Cytotoxic and Anti-HSV-1 Effects of Caulerpin Derivatives
- PMID: 39202939
- PMCID: PMC11357404
- DOI: 10.3390/molecules29163859
Cytotoxic and Anti-HSV-1 Effects of Caulerpin Derivatives
Abstract
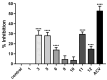
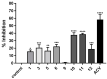
Marine organisms represent a potential source of secondary metabolites with various therapeutic properties. However, the pharmaceutical industry still needs to explore the algological resource. The species Caulerpa lamouroux Forssk presents confirmed biological activities associated with its major compound caulerpin, such as antinociceptive, spasmolytic, antiviral, antimicrobial, insecticidal, and cytotoxic. Considering that caulerpin is still limited, such as low solubility or chemical instability, it was subjected to a structural modifications test to establish which molecular regions could accept structural modification and to elucidate the cytotoxic bioactive structure in Vero cells (African green monkey kidney cells, Cercopithecus aethiops; ATCC, Manassas, VA, USA) and antiviral to Herpes simplex virus type 1. Substitution reactions in the N-indolic position with mono- and di-substituted alkyl, benzyl, allyl, propargyl, and ethyl acetate groups were performed, in addition to conversion to their acidic derivatives. The obtained analogs were submitted to cytotoxicity and antiviral activity screening against Herpes simplex virus type 1 by the tetrazolium microculture method. From the semi-synthesis, 14 analogs were obtained, and 12 are new. The cytotoxicity assay showed that caulerpin acid and N-ethyl-substituted acid presented cytotoxic concentrations referring to 50% of the maximum effect of 1035.0 µM and 1004.0 µM, respectively, values significantly higher than caulerpin. The antiviral screening of the analogs revealed that the N-substituted acids with methyl and ethyl groups inhibited Herpes simplex virus type 1-induced cytotoxicity by levels similar to the positive control acyclovir.
Keywords: Caulerpa racemosa; HSV-1; Vero cells; caulerpin; indolic derivatives.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Sasadhar M., Debjit D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron. 2021;78:131801.
-
- Costa-Lotufo L.V., Wilke D.V., Jimenez P.C. Organismos marinhos como fonte de novos fármacos: Histórico & perspectivas. Quím. Nova. 2009;32:703–716.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

