Caveolae with GLP-1 and NMDA Receptors as Crossfire Points for the Innovative Treatment of Cognitive Dysfunction Associated with Neurodegenerative Diseases
- PMID: 39203005
- PMCID: PMC11357136
- DOI: 10.3390/molecules29163922
Caveolae with GLP-1 and NMDA Receptors as Crossfire Points for the Innovative Treatment of Cognitive Dysfunction Associated with Neurodegenerative Diseases
Abstract
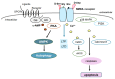
Some neurodegenerative diseases may be characterized by continuing behavioral and cognitive dysfunction that encompasses memory loss and/or apathy. Alzheimer's disease is the most typical type of such neurodegenerative diseases that are characterized by deficits of cognition and alterations of behavior. Despite the huge efforts against Alzheimer's disease, there has yet been no successful treatment for this disease. Interestingly, several possible risk genes for cognitive dysfunction are frequently expressed within brain cells, which may also be linked to cholesterol metabolism, lipid transport, exosomes, and/or caveolae formation, suggesting that caveolae may be a therapeutic target for cognitive dysfunctions. Interestingly, the modulation of autophagy/mitophagy with the alteration of glucagon-like peptide-1 (GLP-1) and N-methyl-d-aspartate (NMDA) receptor signaling may offer a novel approach to preventing and alleviating cognitive dysfunction. A paradigm showing that both GLP-1 and NMDA receptors at caveolae sites may be promising and crucial targets for the treatment of cognitive dysfunctions has been presented here, which may also be able to modify the progression of Alzheimer's disease. This research direction may create the potential to move clinical care toward disease-modifying treatment strategies with maximal benefits for patients without detrimental adverse events for neurodegenerative diseases.
Keywords: Alzheimer’s disease; NMDA; autophagy; caveolae; caveolin; cognitive dysfunction; glucagon-like peptide-1; mitophagy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Caveolae with serotonin and NMDA receptors as promising targets for the treatment of Alzheimer's disease.Int J Physiol Pathophysiol Pharmacol. 2024 Oct 15;16(5):96-110. doi: 10.62347/MTWV3745. eCollection 2024. Int J Physiol Pathophysiol Pharmacol. 2024. PMID: 39583750 Free PMC article. Review.
-
Exosomes, Endosomes, and Caveolae as Encouraging Targets with Favorable Gut Microbiota for the Innovative Treatment of Alzheimer's Diseases.Discov Med. 2024 Nov;36(190):2132-2142. doi: 10.24976/Discov.Med.202436190.196. Discov Med. 2024. PMID: 39600269 Review.
-
Time-dependent effect of oligomeric amyloid-β (1-42)-induced hippocampal neurodegeneration in rat model of Alzheimer's disease.Neurol Res. 2019 Feb;41(2):139-150. doi: 10.1080/01616412.2018.1544745. Epub 2018 Nov 19. Neurol Res. 2019. PMID: 30453864
-
Novel GLP-1 (Glucagon-Like Peptide-1) Analogues and Insulin in the Treatment for Alzheimer's Disease and Other Neurodegenerative Diseases.CNS Drugs. 2015 Dec;29(12):1023-39. doi: 10.1007/s40263-015-0301-8. CNS Drugs. 2015. PMID: 26666230 Review.
-
Heteroreceptor Complexes Formed by Dopamine D1, Histamine H3, and N-Methyl-D-Aspartate Glutamate Receptors as Targets to Prevent Neuronal Death in Alzheimer's Disease.Mol Neurobiol. 2017 Aug;54(6):4537-4550. doi: 10.1007/s12035-016-9995-y. Epub 2016 Jul 1. Mol Neurobiol. 2017. PMID: 27370794
Cited by
-
Ion Channel Regulation in Caveolae and Its Pathological Implications.Cells. 2025 Apr 24;14(9):631. doi: 10.3390/cells14090631. Cells. 2025. PMID: 40358155 Free PMC article. Review.
-
Caveolae-Mediated Transcytosis and Its Role in Neurological Disorders.Biomolecules. 2025 Mar 21;15(4):456. doi: 10.3390/biom15040456. Biomolecules. 2025. PMID: 40305173 Free PMC article. Review.
-
Gut-brain connection in schizophrenia: A narrative review.World J Psychiatry. 2025 May 19;15(5):103751. doi: 10.5498/wjp.v15.i5.103751. eCollection 2025 May 19. World J Psychiatry. 2025. PMID: 40495848 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

