Exclusive Solvent-Controlled Regioselective Catalytic Synthesis of Potentially Bioactive Imidazolidineiminodithiones: NMR Analysis, Computational Studies and X-ray Crystal Structures
- PMID: 39203036
- PMCID: PMC11357535
- DOI: 10.3390/molecules29163958
Exclusive Solvent-Controlled Regioselective Catalytic Synthesis of Potentially Bioactive Imidazolidineiminodithiones: NMR Analysis, Computational Studies and X-ray Crystal Structures
Abstract
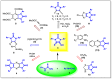
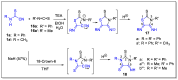
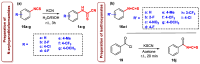
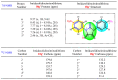
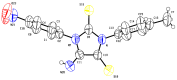
Herein, we describe the first consistent regiospecific reaction of isothiocyanates with a variety of substituted N-arylcyanothioformamides in a 1:1 molar ratio to generate a series of imidazolidineiminodithiones decorated with a multitude of functional groups on both aromatic rings. The reaction is carried out at room temperature using a 20 mol% catalytic amount of triethylamine with DMF as the solvent to selectively form the mentioned products with exclusive regioselectivity. The methodology features wide substrate scope, no requirement for chromatography, and good to high reaction yields. The products were isolated by simple ether/brine extraction and the structures were verified by multinuclear NMR spectroscopy and high accuracy mass measurements. The first conclusive molecular structure elucidation of the observed regioisomer was established by single-crystal X-ray diffraction analysis. Likewise, the tautomer of the N-arylcyanothioformamide reactant was proven by X-ray diffraction analysis. Density functional theory computations at the B3LYP-D4/def2-TZVP level in implicit DMF solvent were conducted to support the noted regiochemical outcome and proposed mechanism.
Keywords: N-arylcyanothioformamides; density functional theory; imidazolidineiminodithiones; isothiocyanates; thiazolidinethiones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Moussa Z., Judeh Z.M.A., Sh El-Sharief A.M. N-arylcyanothioformamides: Preparation methods and application in the synthesis of bioactive molecules. ChemsitrySelect. 2020;5:764–798. doi: 10.1002/slct.201903534. - DOI
-
- Abbas S.Y., Sh El-Sharief, M.A.M.; Salem, M.A.; Sh. El-Sharief, A.M. Utilization of cyanothioformamides in the syntheses of various types of imidazole derivatives. Synth. Commun. 2020;50:621–648. doi: 10.1080/00397911.2019.1700524. - DOI
-
- Olin J.F. Herbicidal Composition and Method Employing Thiooxanilonitriles. 3,287,102. U.S. Patent. 1966 November 22;
-
- Olin J.F., Darlington W.A. Gastropodicidal 3,4-Dichloro-and 3,5-Dichloro-thiooxanilonitriles. 3592911. U.S. Patent. 1971 July 13;
-
- Sh El-Sharief M.A.M., Moussa Z., Sh El-Sharief A.M. Synthesis, characterization, and derivatization of some novel types of fluorinated mono- and bis-imidazolidineiminothiones with antitumor, antiviral, antibacterial, and antifungal activities. J. Fluor. Chem. 2011;132:596–611. doi: 10.1016/j.jfluchem.2011.06.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

