Lactobacillus crispatus-Mediated Gut-Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model
- PMID: 39203401
- PMCID: PMC11356123
- DOI: 10.3390/microorganisms12081559
Lactobacillus crispatus-Mediated Gut-Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model
Abstract
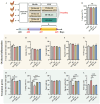
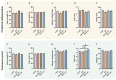
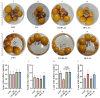
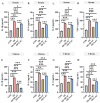
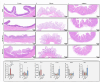
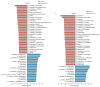
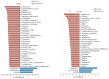
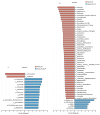
Oviductal inflammation (OI) significantly reduces the egg production and economic returns in poultry farming. While Lactobacillus crispatus (LAC) is effective against inflammation, its role in treating or preventing oviductal inflammation is understudied. In this study, we investigated the therapeutic mechanisms of LAC on oviductal inflammation, with a focus on reproductive tract health, microbiome, gene expression, and cytokine levels. This study involved 24 Jingfen No. 6 laying hens aged 60 weeks, divided into four groups: the CON, OI, OI + LAC, and OI + heat-killed Lactobacillus crispatus (HLAC) groups. And it included a 10-day adaptation, a 7-day period for the development of OI using inflammation-inducing drugs (the control received saline), followed by an 8-day treatment in which the CON and OI groups received 1 mL of MRS broth daily, and the OI + LAC and OI + HLAC groups were treated with live and heat-killed Lactobacillus crispatus (109 CFUs/mL), respectively, with six hens in each group. This study showed that Lactobacillus crispatus supplementation significantly reduced the oviductal inflammation and atrophy in the hens, with the affected hens showing markedly lower egg production rates (p < 0.001) compared to the control and treated groups (OI + HLAC and OI + LAC). The daily intake of fresh (OI + LAC, p = 0.076) or heat-killed (OI + HLAC, p < 0.01) Lactobacillus crispatus notably enhanced the feed conversion efficiency. The OI group suffered significant ovarian damage and vascular rupture, more so than the CON group, while Lactobacillus crispatus supplementation mitigated this damage. The IL-1β, IL-6, and IL-8 levels were significantly elevated in the OI group compared to those in the OI + LAC group (p < 0.05), with a significant reduction in the TNF-α levels in the latter (p < 0.001). The supplementation improved the microbial composition in the cecum, isthmus, and shell gland, enriching the cecum with beneficial bacteria, such as Ruminococcus_torques_group and Megamonas. This approach fostered ovarian health and follicle differentiation and preserved the epithelial cell barrier function in the shell gland, reducing inflammatory damage in the genital tract. This dual efficacy underscores the role of the probiotic in diminishing oviductal inflammation, regardless of its state.
Keywords: Lactobacillus crispatus; gut–reproductive tract axis; hens; laying performance; microbiome; salpingitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Effects of herbal dregs supplementation of Salvia miltiorrhiza and Isatidis Radix residues improved production performance and gut microbiota abundance in late-phase laying hens.Front Vet Sci. 2024 May 3;11:1381226. doi: 10.3389/fvets.2024.1381226. eCollection 2024. Front Vet Sci. 2024. PMID: 38764854 Free PMC article.
-
Possible Toxic Mechanisms of Deoxynivalenol (DON) Exposure to Intestinal Barrier Damage and Dysbiosis of the Gut Microbiota in Laying Hens.Toxins (Basel). 2022 Sep 30;14(10):682. doi: 10.3390/toxins14100682. Toxins (Basel). 2022. PMID: 36287951 Free PMC article.
-
Pine (Pinus massoniana Lamb.) Needle Extract Supplementation Improves Performance, Egg Quality, Serum Parameters, and the Gut Microbiome in Laying Hens.Front Nutr. 2022 Feb 10;9:810462. doi: 10.3389/fnut.2022.810462. eCollection 2022. Front Nutr. 2022. PMID: 35223952 Free PMC article.
-
Rosemary extract improves egg quality by altering gut barrier function, intestinal microbiota and oviductal gene expressions in late-phase laying hens.J Anim Sci Biotechnol. 2023 Sep 4;14(1):121. doi: 10.1186/s40104-023-00904-6. J Anim Sci Biotechnol. 2023. PMID: 37667318 Free PMC article.
-
Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens.Poult Sci. 2022 Feb;101(2):101570. doi: 10.1016/j.psj.2021.101570. Epub 2021 Oct 29. Poult Sci. 2022. PMID: 34852968 Free PMC article.
Cited by
-
Effects of Cyclic Adenosine Monophosphate Nanoliposomes on Growth Performance, Gut Development and Microbiota of Broilers.Animals (Basel). 2025 Jun 23;15(13):1852. doi: 10.3390/ani15131852. Animals (Basel). 2025. PMID: 40646751 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

