Bacillus coagulans LMG S-24828 Impairs Candida Virulence and Protects Vaginal Epithelial Cells against Candida Infection In Vitro
- PMID: 39203476
- PMCID: PMC11356316
- DOI: 10.3390/microorganisms12081634
Bacillus coagulans LMG S-24828 Impairs Candida Virulence and Protects Vaginal Epithelial Cells against Candida Infection In Vitro
Abstract
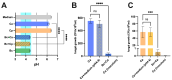
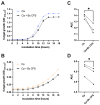
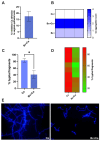
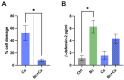
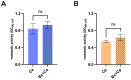
Probiotics are living microbes that provide benefits to the host. The growing data on health promotion, following probiotics administration, increased interest among researchers and pharmaceutical companies. Infections of the lower genital tract in females, caused by a wide range of pathogens, represent one of the main areas for the use of probiotics and postbiotics. Vulvovaginal candidiasis (VVC) affects 75% of women of reproductive age at least once during their lifetime, with 5-8% developing the recurrent form (RVVC). The disease is triggered by the overgrowth of Candida on the vaginal mucosa. Here, in order to establish its probiotic potential in the context of VVC, we evaluated the anti-fungal effects of the spore-producing Bacillus coagulans LMG S-24828 against C. albicans and C. parapsilosis as well as its beneficial effects in counteracting Candida vaginal infection in vitro. Our results show that both live B. coagulans and its Cell-Free Supernatant (CFS) exerted antifungal activity against both fungi. Moreover, live B. coagulans reduced hyphal formation, inhibited C. albicans adhesion to vaginal epithelial cells, showed co-aggregation capacity, and exerted a protective effect on vaginal epithelial cells infected with C. albicans. These data suggest that B. coagulans LMG S-24828 may provide benefits in the context of Candida vaginal infections.
Keywords: Bacillus coagulans; Candida; probiotics; vaginal epithelial cells.
Conflict of interest statement
Author Rosario Russo was employed by the company Giellepi S.p.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro.Microbiol Spectr. 2022 Jun 29;10(3):e0269621. doi: 10.1128/spectrum.02696-21. Epub 2022 May 2. Microbiol Spectr. 2022. PMID: 35499353 Free PMC article.
-
Effectiveness of the association of 2 probiotic strains formulated in a slow release vaginal product, in women affected by vulvovaginal candidiasis: a pilot study.J Clin Gastroenterol. 2012 Oct;46 Suppl:S73-80. doi: 10.1097/MCG.0b013e3182684d71. J Clin Gastroenterol. 2012. PMID: 22955364
-
The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives.Microorganisms. 2023 May 5;11(5):1211. doi: 10.3390/microorganisms11051211. Microorganisms. 2023. PMID: 37317186 Free PMC article. Review.
-
Inhibitory effects of vaginal Lactobacilli on Candida albicans growth, hyphal formation, biofilm development, and epithelial cell adhesion.Front Cell Infect Microbiol. 2023 May 2;13:1113401. doi: 10.3389/fcimb.2023.1113401. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37201113 Free PMC article.
-
The Antibiofilm Role of Biotics Family in Vaginal Fungal Infections.Front Microbiol. 2022 May 26;13:787119. doi: 10.3389/fmicb.2022.787119. eCollection 2022. Front Microbiol. 2022. PMID: 35694318 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

