Alteration of Trophoblast Syncytialization by Plasmodium falciparum-Infected Erythrocytes
- PMID: 39203482
- PMCID: PMC11356531
- DOI: 10.3390/microorganisms12081640
Alteration of Trophoblast Syncytialization by Plasmodium falciparum-Infected Erythrocytes
Abstract
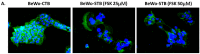
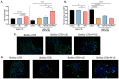
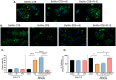
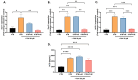
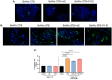
Malaria during pregnancy has been associated with significant risks to both the mother and the fetus, leading to complications such as anemia, low birth weight, and increased infant mortality. The trophoblast cells, a key component of the placenta, are crucial for nutrient and oxygen exchange between mother and fetus. The differentiation of cytotrophoblasts (CTBs) into syncytiotrophoblasts (STBs) is critical for proper pregnancy development. These cells form the bi-stratified epithelium surrounding the placental villi. While previous studies have described an inflammatory activation of STB cells exposed to Plasmodium falciparum-infected erythrocytes (P. falciparum-IE) or components such as hemozoin (HZ), little is known about the direct effect this parasite may have on the epithelial turnover and function of trophoblast cells. This study aims to contribute to understanding mechanisms leading to placental damage during placental malaria using a BeWo cell line as a differentiation model. It was found that P. falciparum-IE interferes with the fusion of BeWo cells, affecting the differentiation process of trophoblast. A reduction in syncytialization could be associated with the adverse effects of infection in fetal health, altering the remodeling of the trophoblast epithelial barrier and reducing their capacity to exchange substances. However, further studies are necessary to assess alterations in the functionality of this epithelium.
Keywords: Plasmodium falciparum; cytotrophoblast; malaria; syncytiotrophoblast; trophoblast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fried M., Kurtis J.D., Swihart B., Pond-Tor S., Barry A., Sidibe Y., Gaoussou S., Traore M., Keita S., Mahamar A., et al. Systemic inflammatory response to malaria during pregnancy is associated with pregnancy loss and preterm delivery. Clin. Infect. Dis. 2017;65:1729–1735. doi: 10.1093/cid/cix623. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

