Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection
- PMID: 39203489
- PMCID: PMC11357294
- DOI: 10.3390/microorganisms12081647
Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection
Abstract
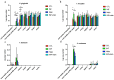
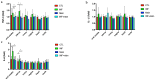
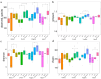
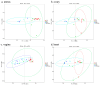
Periodontitis-related oral microbial dysbiosis is thought to contribute to adverse pregnancy outcomes (APOs), infertility, and female reproductive inflammation. Since probiotics can modulate periodontitis and oral microbiome dysbiosis, this study examined the effects of a probiotic bacteriocin, nisin, in modulating the reproductive microbiome and inflammation triggered by periodontitis. A total of 24 eight-week-old BALB/cByJ female mice were randomly divided into four treatment groups (control, infection, nisin, and infection+nisin group), with 6 mice per group. A polymicrobial (Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Fusobacterium nucleatum) mouse model of periodontal disease was used to evaluate the effects of this disease on the female reproductive system, with a focus on the microbiome, local inflammation, and nisin's therapeutic potential in this context. Moreover, 16s RNA sequencing was used to evaluate the changes in the microbiome and RT-PCR was used to evaluate the changes in inflammatory cytokines. Periodontal pathogen DNA was detected in the reproductive organs, and in the heart and aorta at the end of the experimental period, and the DNA was especially elevated in the oral cavity in the infection group. Compared to the control groups, only P. gingivalis was significantly higher in the oral cavity and uterus of the infection groups, and T. forsythia and F. nucleatum were significantly higher in the oral cavity of the infection groups. The infection and nisin treatment group had significantly lower levels of P. gingivalis, T. forsythia, and F. nucleatum in the oral cavity compared with the infection group. Since periodontal pathogen DNA was also detected in the heart and aorta, this suggests potential circulatory system transmission. The polymicrobial infection generally decreased the microbiome diversity in the uterus, which was abrogated by nisin treatment. The polymicrobial infection groups, compared to the control groups, generally had lower Firmicutes and higher Bacteroidota in all the reproductive organs, with similar trends revealed in the heart. However, the nisin treatment group and the infection and nisin group, compared to the control or infection groups, generally had higher Proteobacteria and lower Firmicutes and Bacteroidota in the reproductive organs and the heart. Nisin treatment also altered the microbiome community structure in the reproductive tract to a new state that did not mirror the controls. Periodontal disease, compared to the controls, triggered an increase in inflammatory cytokines (IL-6, TNF-α) in the uterus and oral cavity, which was abrogated by nisin treatment. Polymicrobial periodontal disease alters the reproductive tract's microbial profile, microbiome, and inflammatory status. Nisin modulates the microbial profile and microbiome of the reproductive tract and mitigates the elevated uterine inflammatory cytokines triggered by periodontal disease.
Keywords: antimicrobial therapy; nisin; oral microbiome; periodontal disease; reproductive tract microbiome.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

