Exploring the Role of Bergamot Polyphenols in Alleviating Morphine-Induced Hyperalgesia and Tolerance through Modulation of Mitochondrial SIRT3
- PMID: 39203757
- PMCID: PMC11357234
- DOI: 10.3390/nu16162620
Exploring the Role of Bergamot Polyphenols in Alleviating Morphine-Induced Hyperalgesia and Tolerance through Modulation of Mitochondrial SIRT3
Abstract
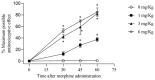
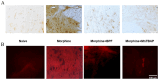
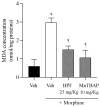
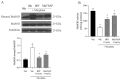
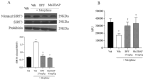
Morphine is an important pain reliever employed in pain management, its extended utilize is hindered by the onset of analgesic tolerance and oxidative stress. Long-term morphine administration causes elevated production of reactive oxygen species (ROS), disrupting mitochondrial function and inducing oxidation. Sirtuin 3 (SIRT3), a mitochondrial protein, is essential in modulating ROS levels by regulating mitochondrial antioxidant enzymes as manganese superoxide dismutase (MnSOD). Our investigation focused on the impact of SIRT3 on hyperalgesia and morphine tolerance in mice, as evaluating the antioxidant effect of the polyphenolic fraction of bergamot (BPF). Mice were administered morphine twice daily for four consecutive days (20 mg/kg). On the fifth day, mice received an acute dose of morphine (3 mg/kg), either alone or in conjunction with BPF or Mn (III)tetrakis (4-benzoic acid) porphyrin (MnTBAP). We evaluated levels of malondialdehyde (MDA), nitration, and the activity of SIRT3, MnSOD, glutamine synthetase (GS), and glutamate 1 transporter (GLT1) in the spinal cord. Our findings demonstrate that administering repeated doses of morphine led to the development of antinociceptive tolerance in mice, accompanied by increased superoxide production, nitration, and inactivation of mitochondrial SIRT3, MnSOD, GS, and GLT1. The combined administration of morphine with either BPF or MnTBAP prevented these effects.
Keywords: Sirtuin 3; antioxidants; hyperalgesia; mitochondrial dysfunction; morphine-induced tolerance; oxidative stress; polyphenols; therapeutic approach.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Birkinshaw H., Friedrich C.M., Cole P., Eccleston C., Serfaty M., Stewart G., White S., Moore R.A., Phillippo D., Pincus T. Antidepressants for pain management in adults with chronic pain: A network meta-analysis. Cochrane Database Syst. Rev. 2023;5:CD014682. doi: 10.1002/14651858.CD014682.pub2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

