Nicotinamide Suppresses Hyperactivation of Dendritic Cells to Control Autoimmune Disease through PARP Dependent Signaling
- PMID: 39203802
- PMCID: PMC11356829
- DOI: 10.3390/nu16162665
Nicotinamide Suppresses Hyperactivation of Dendritic Cells to Control Autoimmune Disease through PARP Dependent Signaling
Abstract
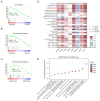
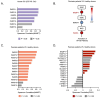
Dendritic cells (DCs) are crucial in initiating and shaping both innate and adaptive immune responses. Clinical studies and experimental models have highlighted their significant involvement in various autoimmune diseases, positioning them as promising therapeutic targets. Nicotinamide (NAM), a form of vitamin B3, with its anti-inflammatory properties, has been suggested, while the involvement of NAM in DCs regulation remains elusive. Here, through analyzing publicly available databases, we observe substantial alterations in NAM levels and NAM metabolic pathways during DCs activation. Furthermore, we discover that NAM, but not Nicotinamide Mononucleotide (NMN), significantly inhibits DCs over-activation in vitro and in vivo. The suppression of DCs hyperactivation effectively alleviates symptoms of psoriasis. Mechanistically, NAM impairs DCs activation through a Poly (ADP-ribose) polymerases (PARPs)-NF-κB dependent manner. Notably, phosphoribosyl transferase (NAMPT) and PARPs are significantly upregulated in lipopolysaccharide (LPS)-stimulated DCs and psoriasis patients; elevated NAMPT and PARPs expression in psoriasis patients correlates with higher psoriasis area and severity index (PASI) scores. In summary, our findings underscore the pivotal role of NAM in modulating DCs functions and autoimmune disorders. Targeting the NAMPT-PARP axis emerges as a promising therapeutic approach for DC-related diseases.
Keywords: NF-κB; PARP; dendritic cell; nicotinamide; psoriasis.
Conflict of interest statement
All authors declared that this work was performed without any conflicts of interest.
Figures





References
-
- Yuan X., Duan Y., Xiao Y., Sun K., Qi Y., Zhang Y., Ahmed Z., Moiani D., Yao J., Li H., et al. Vitamin E Enhances Cancer Immunotherapy by Reinvigorating Dendritic Cells via Targeting Checkpoint SHP1. Cancer Discov. 2022;12:1742–1759. doi: 10.1158/2159-8290.CD-21-0900. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

