Immunogenicity of an Extended Dose Interval for the Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen in Adults and Children in the Democratic Republic of the Congo
- PMID: 39203955
- PMCID: PMC11359010
- DOI: 10.3390/vaccines12080828
Immunogenicity of an Extended Dose Interval for the Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen in Adults and Children in the Democratic Republic of the Congo
Abstract
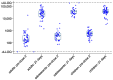
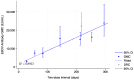
During the 2018-2020 Ebola virus disease outbreak in Democratic Republic of the Congo, a phase 3 trial of the Ad26.ZEBOV, MVA-BN-Filo Ebola vaccine (DRC-EB-001) commenced in Goma, with participants being offered the two-dose regimen given 56 days apart. Suspension of trial activities in 2020 due to the COVID-19 pandemic led to some participants receiving a late dose 2 outside the planned interval. Blood samples were collected from adults, adolescents, and children prior to their delayed dose 2 vaccination and 21 days after, and tested for IgG binding antibodies against Ebola virus glycoprotein using the Filovirus Animal Nonclinical Group (FANG) ELISA. Results from 133 participants showed a median two-dose interval of 9.3 months. The pre-dose 2 antibody geometric mean concentration (GMC) was 217 ELISA Units (EU)/mL (95% CI 157; 301) in adults, 378 EU/mL (281; 510) in adolescents, and 558 EU/mL (471; 661) in children. At 21 days post-dose 2, the GMC increased to 22,194 EU/mL (16,726; 29,449) in adults, 37,896 EU/mL (29,985; 47,893) in adolescents, and 34,652 EU/mL (27,906; 43,028) in children. Participants receiving a delayed dose 2 had a higher GMC at 21 days post-dose 2 than those who received a standard 56-day regimen in other African trials, but similar to those who received the regimen with an extended interval.
Keywords: Ad26.ZEBOV; DRC; Democratic Republic of the Congo; Ebola; MVA-BN-Filo; Mvabea; Zabdeno; immunogenicity; interval; outbreak; vaccine.
Conflict of interest statement
The funders advised on the study design and protocol, but had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Janssen Vaccines & Prevention B.V. was the vaccine manufacturer and donated the vaccine for this study. B.K., A.G., C.M., K.L., and C.R. were full-time employees of Janssen, Pharmaceutical Companies of Johnson & Johnson at the time of the study. A.G., C.M., K.L., and C.R. declared ownership of shares in Janssen, Pharmaceutical Companies of Johnson & Johnson. D.W.-J. and Janssen Vaccines & Prevention B.V. are consortium members for the EBOVAC1 and EBOVAC3 projects. All other authors declare no conflicts of interests.
Figures


References
-
- World Health Organization Ebola Outbreak 2018–2020 North Kivu-Ituri. 2020. [(accessed on 16 August 2023)]. Available online: https://www.who.int/emergencies/situations/Ebola-2019-drc-
-
- European Medicines Agency Ervebo Ebola Zaire Vaccine (rVSVG-ZEBOV-GP, Live) Dec 12, 2019. [(accessed on 16 August 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo.
-
- World Health Organization . Strategic Advisory Group of Experts (SAGE) on Immunization Interim Recommendations on Vaccination against Ebola Virus Disease (EVD) World Health Organization; Geneva, Switzerland: May 7, 2019. [(accessed on 16 August 2023)]. Available online: https://cdn.who.int/media/docs/default-source/immunization/ebola/interim....
-
- Watson-Jones D., Kavunga-Membo H., Grais R.F., Ahuka S., Roberts N., Edmunds W.J., Choi E.M., Roberts C.H., Edwards T., Camacho A., et al. Protocol for a phase 3 trial to evaluate the effectiveness and safety of a heterologous, two-dose vaccine for Ebola virus disease in the Democratic Republic of the Congo. BMJ Open. 2022;12:e055596. doi: 10.1136/bmjopen-2021-055596. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

