Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine
- PMID: 39203990
- PMCID: PMC11359048
- DOI: 10.3390/vaccines12080864
Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine
Abstract
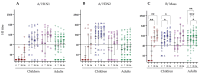
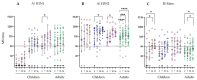
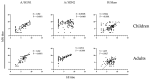
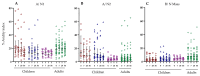
Live attenuated influenza vaccines (LAIV) typically induce a poor hemagglutination inhibition (HI) response, which is the standard correlate of protection for inactivated influenza vaccines. The significance of the HI response is complicated because the LAIV vaccine primarily induces the local mucosal immune system, while the HI assay measures the circulating serum antibody response. However, age and pre-existing immunity have been identified as important factors affecting LAIV immunogenicity. This study aimed to extend our understanding of LAIV-induced immunity, particularly, the impact age and pre-existing immunity have on eliciting functional and neutralising antibody responses in paediatric and adult populations vaccinated with LAIV. Thirty-one children and 26 adults were immunized with the trivalent LAIV during the 2013-2014 influenza season in Norway. Children under 9 years received a second dose of LAIV 28 days after the first dose. Blood samples were collected pre- and post-vaccination. HI, microneutralization (MN) and enzyme-linked lectin assay for neuraminidase (NA) antibodies were measured against the vaccine strains. IgG antibody avidity against hemagglutinin (HA) and NA proteins from the vaccine strains was also assessed. Significant correlations were observed between HI and MN responses to A/California/7/2009 (A/H1N1)pdm09-like strain and B/Massachusetts/2/2012-like strain, suggesting that MN is a potential immunological correlate for LAIV. However, the relationship between recipient age (or priming status) and serological response varied between vaccine strains. There was a notable increase in HI and MN responses in all cohorts except naive children against the H1N1 strain, where most recipients had responses below the protective antibody threshold. NAI responses were generally weak in naive children against all vaccine strains compared with adults or antigen-primed children. Post-vaccination antibody avidity increased only in primed children below nine years of age against the A/H1N1 strain. Overall, our findings indicate that LAIV elicits functional and neutralizing antibody responses in both naive and antigen experienced cohorts, however, the magnitude and kinetics of the response varies between vaccine strains.
Keywords: adults; children; functional antibodies; immune response; live attenuated influenza vaccine.
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines, influenza virus vaccines, and influenza virus therapeutics which list Florian Krammer as a co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, CastleVax, to develop SARS-CoV-2 vaccines. Florian Krammer is a co-founder and scientific advisory board member of CastleVax. Florian Krammer has consulted for Merck, GSK, Curevac, Seqirus, and Pfizer, and is currently consulting for 3rd Rock Ventures, Gritstone, and Avimex. The Krammer laboratory is collaborating with Dynavax on influenza vaccine development. The other authors declare no conflicts of interest.
Figures


References
-
- Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Estimates of global seasonal influenza-associated respiratory mortality: A modeling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control Factsheet about Seasonal Influenza. 14 June 2017. [(accessed on 30 July 2024)]. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet.
-
- Centers for Disease Control and Prevention Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine) [(accessed on 30 July 2024)];2024 Available online: https://www.cdc.gov/flu/prevent/nasalspray.htm.
-
- Belshe R.B., Edwards K.M., Vesikari T., Black S.V., Walker R.E., Hultquist M., Kemble G., Connor E.M. CAIV-T Comparative Efficacy Study Group Live attenuated versus inactivated influenza vaccine in infants and young children. New Engl. J. Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. - DOI - PubMed
-
- Fleming D.M., Crovari P., Wahn U., Klemola T., Schlesinger Y., Langussis A., Øymar K., Garcia M.L., Krygier A., Costa H., et al. Comparison of the Efficacy and Safety of Live Attenuated Cold-Adapted Influenza Vaccine, Trivalent, with Trivalent Inactivated Influenza Virus Vaccine in Children and Adolescents with Asthma. Pediatr. Infect. Dis. J. 2006;25:860–869. doi: 10.1097/01.inf.0000237797.14283.cf. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

