Tixagevimab/Cilgavimab for COVID-19 Pre-Exposure Prophylaxis in Hematologic Patients-A Tailored Approach Based on SARS-CoV-2 Vaccine Response
- PMID: 39203997
- PMCID: PMC11359694
- DOI: 10.3390/vaccines12080871
Tixagevimab/Cilgavimab for COVID-19 Pre-Exposure Prophylaxis in Hematologic Patients-A Tailored Approach Based on SARS-CoV-2 Vaccine Response
Abstract
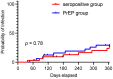
Patients with hematologic malignancies still face a significant risk of severe coronavirus disease 2019 (COVID-19). The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)-neutralizing monoclonal antibody combination tixagevimab/cilgavimab (TIX/CGB) could be administered to immunocompromised patients for pre-exposure prophylaxis (PrEP) before the emergence of TIX/CGB-resistant COVID-19 Omicron variants. TIX/CGB application could be carried out regardless of the host's immune response to previous active SARS-CoV-2 vaccinations or infections. Because the efficacy of COVID-19 PrEP remains unclear, especially in SARS-CoV-2-seropositive patients, German national guidelines recommended TIX/CGB PrEP only for SARS-CoV-2-seronegative patients in addition to an intensified active vaccination schedule. Having followed these guidelines, we now report the characteristics and outcomes of 54 recipients of TIX/CGB PrEP in SARS-CoV-2-seronegative patients with hematological disease from a German tertiary medical center and compare them to 125 seropositive patients who did not receive any PrEP. While the number of patients with B-cell lymphomas was significantly higher in the seronegative cohort (33 (61%) vs. 18 (14%) cases, p < 0.01), patients with myeloid diseases were significantly more frequent in the seropositive cohort (51 (41%) vs. 5 (9%) cases, p < 0.01). Strikingly, patients who had undergone allogeneic hematopoietic stem cell transplantation were significantly more likely (forty-nine (39%) vs. six (11%) cases, p < 0.01) to be SARS-CoV-2 seropositive. We observed that prophylactic application of TIX/CGB PrEP to a highly vulnerable group of SARS-CoV-2-seronegative patients resulted in a similar number of COVID-19 breakthrough infections compared to the untreated seropositive control group (16 (32%) vs. 39 (36%), p = 0.62) and comparable COVID-19-related outcomes like hospitalization and oxygen requirement throughout an extended follow-up period of 12 months. In conclusion, our results support the tailored approach of administering TIX/CGB PrEP only to SARS-CoV-2-seronegative patients during the COVID-19 pandemic and might provide a rationale for similar strategies during future outbreaks/diseases, especially in times of initial limited availability and/or financial constraints.
Keywords: COVID-19; PrEP; hematologic malignancy; neutralizing antibodies; tixagevimab/cilgavimab.
Conflict of interest statement
C.D.S. reports grants and personal fees from AstraZeneca, personal fees and non-financial support from Braun Melsungen, personal fees from BioNtech, grants, personal fees and non-financial support from Gilead Sciences, grants and personal fees from Janssen-Cilag, personal fees from Eli Lilly, personal fees from Formycon, personal fees from Pfizer, personal fees from Roche, other grants from Apeiron, grants and personal fees from MSD, grants from Cepheid, personal fees from GSK, personal fees from Molecular Partners, personal fees from Pfizer, other grants from Eli Lilly, personal fees from SOBI during this study, personal fees from AbbVie, personal fees from MSD, personal fees from Synairgen, personal fees from Shionogi, and grants and personal fees from ViiV Healthcare outside the submitted work. FB reports personal fees from BMS and Janssen.
Figures



References
-
- Bilich T., Roerden M., Maringer Y., Nelde A., Heitmann J.S., Dubbelaar M.L., Peter A., Horber S., Bauer J., Rieth J., et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021;11:1982–1995. doi: 10.1158/2159-8290.CD-21-0191. - DOI - PubMed
-
- Passamonti F., Romano A., Salvini M., Merli F., Porta M.G.D., Bruna R., Coviello E., Romano I., Cairoli R., Lemoli R., et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br. J. Haematol. 2021;195:371–377. doi: 10.1111/bjh.17704. - DOI - PMC - PubMed
-
- Piechotta V., Mellinghoff S.C., Hirsch C., Brinkmann A., Iannizzi C., Kreuzberger N., Adams A., Monsef I., Stemler J., Cornely O.A., et al. Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: A systematic review. Blood Cancer J. 2022;12:86. doi: 10.1038/s41408-022-00684-8. - DOI - PMC - PubMed
-
- Blennow O., Salmanton-Garcia J., Nowak P., Itri F., Van Doesum J., Lopez-Garcia A., Farina F., Jaksic O., Pinczes L.I., Bilgin Y.M., et al. Outcome of infection with omicron SARS-CoV-2 variant in patients with hematological malignancies: An EPICOVIDEHA survey report. Am. J. Hematol. 2022;97:E312–E317. doi: 10.1002/ajh.26626. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

