Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 39204005
- PMCID: PMC11360664
- DOI: 10.3390/vaccines12080879
Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Background: Respiratory syncytial virus (RSV) is garnering increasing attention, with a growing number of subunit RSV vaccines under active clinical investigation. However, comprehensive evidence is limited.
Methods: We conducted a comprehensive search across PubMed, Embase, the Cochrane Library, Web of Science, and ClinicalTrials.gov from database inception to 12 January 2024, focusing on published randomized controlled trials (RCTs).
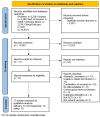
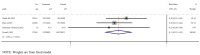
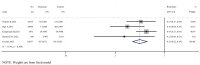
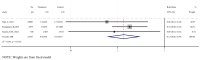
Results: A total of 17 studies were included, encompassing assessments of efficacy (5 studies), safety (17 studies), and immunogenicity (12 studies) of RSV subunit vaccines. The pooled risk ratio (RR) for RSV-associated acute respiratory infection (RSV-ARI) with subunit vaccines was 0.31 (95% CI: 0.23-0.43), for RSV-associated lower respiratory tract infection (RSV-LRTI), it was 0.32 (95% CI: 0.22-0.44), and for severe RSV-LRTI (RSV-SLRTI), it was 0.13 (95% CI: 0.06-0.29). There was no significant difference in serious adverse events (SAEs) between the vaccine and placebo groups, with a pooled RR of 1.05 (95% CI: 0.98-1.14). The pooled standardized mean difference (SMD) for the geometric mean titer (GMT) of neutralizing antibodies was 2.89 (95% CI: 2.43-3.35).
Conclusion: Subunit RSV vaccines exhibit strong efficacy, favorable safety profiles, and robust immunogenicity. Future research should focus on the cost-effectiveness of various vaccines to enhance regional and national immunization strategies.
Keywords: efficacy; immunogenicity; meta-analysis; respiratory syncytial virus vaccines; safety.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures


Similar articles
-
Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis.BMC Public Health. 2024 May 6;24(1):1244. doi: 10.1186/s12889-024-18748-8. BMC Public Health. 2024. PMID: 38711074 Free PMC article.
-
Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study.Vaccine. 2019 May 6;37(20):2694-2703. doi: 10.1016/j.vaccine.2019.04.011. Epub 2019 Apr 12. Vaccine. 2019. PMID: 30987852 Clinical Trial.
-
Efficacy and safety of respiratory syncytial virus vaccination during pregnancy to prevent lower respiratory tract illness in newborns and infants: a systematic review and meta-analysis of randomized controlled trials.Front Pediatr. 2024 Jan 31;11:1260740. doi: 10.3389/fped.2023.1260740. eCollection 2023. Front Pediatr. 2024. PMID: 38357264 Free PMC article. Review.
-
Respiratory syncytial virus vaccine: a systematic overview with emphasis on respiratory syncytial virus subunit vaccines.Vaccine. 2001 Dec 12;20(5-6):954-60. doi: 10.1016/s0264-410x(01)00388-7. Vaccine. 2001. PMID: 11738763
-
Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: Results of a randomized phase 3 study.Vaccine. 2024 May 10;42(13):3172-3179. doi: 10.1016/j.vaccine.2024.03.070. Epub 2024 Apr 16. Vaccine. 2024. PMID: 38616438 Clinical Trial.
Cited by
-
Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients.Epidemiologia (Basel). 2025 Apr 1;6(2):16. doi: 10.3390/epidemiologia6020016. Epidemiologia (Basel). 2025. PMID: 40265347 Free PMC article.
References
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Savic M., Penders Y., Shi T., Branche A., Pirçon J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses. 2023;17:e13031. doi: 10.1111/irv.13031. - DOI - PMC - PubMed
-
- Gill C.J., Mwananyanda L., MacLeod W.B., Kwenda G., Pieciak R., Mupila Z., Murphy C., Chikoti C., Forman L., Berklein F. Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: A 3-year, systematic, post-mortem surveillance project. Lancet Glob. Health. 2022;10:e269–e277. doi: 10.1016/S2214-109X(21)00518-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

