Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine
- PMID: 39204029
- PMCID: PMC11360114
- DOI: 10.3390/vaccines12080903
Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine
Abstract
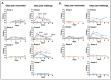
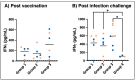
Human monocytic ehrlichiosis, an emerging tick-borne disease, is caused by Ehrlichia chaffeensis. Infections with the pathogen are also common in the canine host. Our previous studies demonstrated that functional disruption within the E. chaffeensis phage head-to-tail connector protein gene results in bacterial attenuation, creating a modified live attenuated vaccine (MLAV). The MLAV confers protective immunity against intravenous and tick transmission challenges one month following vaccination. In this study, we evaluated the duration of MLAV protection. Dogs vaccinated with the MLAV were challenged with wild-type E. chaffeensis via intravenous infection at 4-, 8-, and 12-months post-vaccination. Immunized dogs rapidly cleared the wild-type pathogen infection and tested positive for bacteremia less frequently than unvaccinated controls. While immune responses varied among dogs, vaccinees consistently mounted IgG and CD4+ T-cell responses specific to E. chaffeensis throughout the assessment period. Our findings demonstrate that MLAV-mediated immune protection persists for at least one year against wild-type bacterial infection, marking a major advancement in combating this serious tick-borne disease. The data presented here serve as the foundation for further studies, elucidating the molecular mechanisms underlying virulence and vaccine development and aiding in preventing the diseases caused by E. chaffeensis and other tick-borne rickettsial pathogens.
Keywords: Ehrlichia chaffeensis; immune protection; long-lasting immunity; modified live vaccine; monocytic ehrlichiosis; rickettsiales; tick-borne disease.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Breitschwerdt E.B., Hegarty B.C., Qurollo B.A., Saito T.B., Maggi R.G., Blanton L.S., Bouyer D.H. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors. 2014;7:1756–3305. doi: 10.1186/1756-3305-7-298. - DOI - PMC - PubMed
Grants and funding
- R01 AI070908/AI/NIAID NIH HHS/United States
- R01 AI152418/AI/NIAID NIH HHS/United States
- R56 AI070908/AI/NIAID NIH HHS/United States
- AI152418 and AI070908/This research was funded by PHS grants AI152418 and AI070908 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
LinkOut - more resources
Full Text Sources
Research Materials

