COVID-19 Vaccine Effectiveness Studies against Symptomatic and Severe Outcomes during the Omicron Period in Four Countries in the Eastern Mediterranean Region
- PMID: 39204033
- PMCID: PMC11360574
- DOI: 10.3390/vaccines12080906
COVID-19 Vaccine Effectiveness Studies against Symptomatic and Severe Outcomes during the Omicron Period in Four Countries in the Eastern Mediterranean Region
Abstract
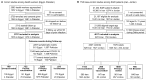
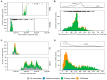
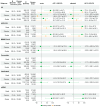
Vaccine effectiveness (VE) studies provide real-world evidence to monitor vaccine performance and inform policy. The WHO Regional Office for the Eastern Mediterranean supported a regional study to assess the VE of COVID-19 vaccines against different clinical outcomes in four countries between June 2021 and August 2023. Health worker cohort studies were conducted in 2707 health workers in Egypt and Pakistan, of whom 171 experienced symptomatic laboratory-confirmed SARS-CoV-2 infection. Test-negative design case-control studies were conducted in Iran and Jordan in 4017 severe acute respiratory infection (SARI) patients (2347 controls and 1670 cases) during the Omicron variant dominant period. VE estimates were calculated for each study and pooled by study design for several vaccine types (BBIBP-CorV, AZD1222, BNT162b2, and mRNA-1273, among others). Among health workers, VE against symptomatic infection of a complete primary series could only be computed compared to partial vaccination, suggesting a benefit of providing an additional dose of mRNA vaccines (VE: 88.9%, 95%CI: 15.3-98.6%), while results were inconclusive for other vaccine products. Among SARI patients, VE against hospitalization of a complete primary series with any vaccine compared to non-vaccinated was 20.9% (95%CI: 4.5-34.5%). Effectiveness estimates for individual vaccines, booster doses, and secondary outcomes (intensive care unit admission and death) were inconclusive. Future VE studies will need to address challenges in both design and analysis when conducted late during a pandemic and will be able to utilize the strengthened capacities in countries.
Keywords: COVID-19; Omicron; SARS-CoV-2; eastern Mediterranean region; vaccine effectiveness.
Conflict of interest statement
No competing or conflicting interest are declared.
Figures


References
-
- Meslé M.M., Brown J., Mook P., Hagan J., Pastore R., Bundle N., Spiteri G., Ravasi G., Nicolay N., Andrews N., et al. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Eurosurveillance. 2021;26:2101021. doi: 10.2807/1560-7917.ES.2021.26.47.2101021. - DOI - PMC - PubMed
-
- WHO R&D Blueprint and COVID-19. 2023. [(accessed on 3 April 2024)]. Available online: https://www.who.int/teams/blueprint/covid-19.
-
- WHO Policy Brief: Reaching COVID-19 Vaccination Targets. 2022. [(accessed on 3 April 2024)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy_Brief-Vacci....
-
- Kheirandish M., Karimian Z., Fahmy K., Rashidian A., Hajjeh R. Capacity-building for conducting COVID-19 vaccine effectiveness studies to enhance evidence-informed vaccination policymaking in the Eastern Mediterranean Region. East Mediterr. Health J. 2023;29:562–569. doi: 10.26719/emhj.23.094. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

