Vaccines for Respiratory Viruses-COVID and Beyond
- PMID: 39204059
- PMCID: PMC11360283
- DOI: 10.3390/vaccines12080936
Vaccines for Respiratory Viruses-COVID and Beyond
Abstract
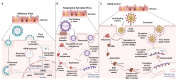
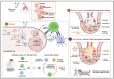
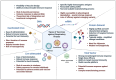
The COVID-19 (coronavirus disease 2019) pandemic had an extensive impact on global morbidity and mortality. Several other common respiratory viruses, such as the influenza virus and respiratory syncytial virus (RSV), are endemic or epidemic agents causing acute respiratory infections that are easily transmissible and pose a significant threat to communities due to efficient person-to-person transmission. These viruses can undergo antigenic variation through genetic mutations, resulting in the emergence of novel strains or variants, thereby diminishing the effectiveness of current vaccines, and necessitating ongoing monitoring and adjustment of vaccine antigens. As the virus-specific immunity is maintained only for several weeks or months after the infection, there is an emergent need to develop effective and durable vaccines. Additionally, specific populations, such as elderly or immunocompromised individuals, may exhibit reduced immune responses to respiratory viruses, posing significant challenges to develop vaccines that elicit durable and potent immunity. We present a comprehensive review of the molecular mechanisms underlying the pathogenesis and virulence of common respiratory viruses, such as RSV, influenza virus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We discuss several vaccine approaches that are under development. A thorough understanding of the current strategies and the challenges encountered during the vaccine development process can lead to the advancement of effective next-generation vaccines.
Keywords: SARS-CoV-2; acute respiratory infections; influenza virus; respiratory syncytial virus; vaccine development.
Conflict of interest statement
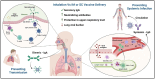
The authors are employees at Ocugen Inc., which is currently developing inhalation vaccines for COVID-19 and flu.
Figures
References
-
- Petrova V.N., Russell C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018;16:60. - PubMed
-
- World Health Organization Influenza (Seasonal); Fact Sheet No. 211. [(accessed on 19 August 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

