Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever
- PMID: 39204071
- PMCID: PMC11360710
- DOI: 10.3390/vaccines12080948
Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever
Abstract
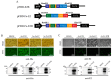
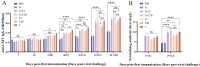
The occurrence of classical swine fever (CSF) poses a significant threat to the global swine industry. Developing an effective and safe vaccine is crucial for preventing and controlling CSF. Here, we constructed self-assembled ferritin nanoparticles fused with the classical swine fever virus (CSFV) E2 protein and a derived B cell epitope (Fe-E2B) using a baculovirus expression system (BVES), demonstrating enhanced immunogenicity. Furthermore, we provide a detailed evaluation of the immunological efficacy of the FeE2B in rabbits. The results showed that robust and sustained antibody responses were detected in rabbits immunized with the Fe-E2B nanoparticle vaccine, comparable to those elicited by commercially available vaccines. Additionally, we demonstrated that the vaccine effectively activated crucial immune factors IFN-γ and IL-4 in vivo, increasing their levels by 1.41-fold and 1.39-fold, respectively. Immunization with Fe-E2B enabled rabbits to avoid viremia and stereotypic fever after CSFV challenge. In conclusion, this study highlights the potential of ferritin nanoparticles as antigen-presenting carriers to induce robust immune responses, proposing a candidate vaccine strategy for the prevention and control of CSF.
Keywords: B-cell antigenic epitope; E2 protein; classical swine fever virus; ferritin nanoparticles; immune responses.
Conflict of interest statement
The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Ji W., Guo Z., Ding N.Z., He C.Q. Studying classical swine fever virus: Making the best of a bad virus. Virus Res. 2015;197:35–47. - PubMed
-
- Becher P., Ramirez R.A., Orlich M., Rosales S.C., König M., Schweizer M., Stalder H., Schirrmeier H., Thiel H.J. Genetic and antigenic characterization of novel pestivirus genotypes: Implications for classification. Virology. 2003;311:96–104. - PubMed
-
- Lohse L., Nielsen J., Uttenthal S. Early pathogenesis of classical swine fever virus (CSFV) strains in Danish pigs. Vet. Microbiol. 2012;159:327–336. - PubMed
Grants and funding
- 32172824 and 32102643/National Natural Science Foundation of China under Grant
- 202201010489/Guangzhou Basic and Applied Basic Research Program
- 202206010161/Guangzhou Science and Technology Program
- C18/Quality Improvement Project of South China Agricultural University
- WS-HN-JKYZ-202404-118/South China Agricultural University and Wen's Group Science and Technology Innovation Centre Results in Incubation Project
LinkOut - more resources
Full Text Sources

