Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro
- PMID: 39204085
- PMCID: PMC11356971
- DOI: 10.3390/ph17080980
Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro
Abstract
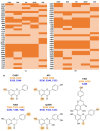
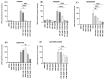
The molecular mechanisms underlying the observed anticancer effects of flavonoids remain unclear. Increasing evidence shows that the aryl hydrocarbon receptor (AHR) plays a crucial role in neoplastic disease progression, establishing it as a potential drug target. This study evaluated the potential of hydroxy flavonoids, known for their anticancer properties, to interact with AHR, both in silico and in vitro, aiming to understand the mechanisms of action and identify selective AHR modulators. A PAS-B domain homology model was constructed to evaluate in silico interactions of chrysin, naringenin, quercetin apigenin and agathisflavone. The EROD activity assay measured the effects of flavonoids on AHR's activity in human breast cancer cells (MCF7). Simulations showed that chrysin, apigenin, naringenin, and quercetin have the highest AHR binding affinity scores (-13.14 to -15.31), while agathisflavone showed low scores (-0.57 and -5.14). All tested flavonoids had the potential to inhibit AHR activity in a dose-dependent manner in the presence of an agonist (TCDD) in vitro. This study elucidates the distinct modulatory effects of flavonoids on AHR, emphasizing naringenin's newly described antagonistic potential. It underscores the importance of understanding flavonoid's molecular mechanisms, which is crucial for developing novel cancer therapies based on these molecules.
Keywords: AHR; TCDD; antagonist; cancer therapy; naringenin; polyphenols.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

