In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability
- PMID: 39204093
- PMCID: PMC11357342
- DOI: 10.3390/ph17080988
In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability
Abstract
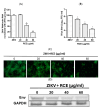
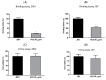
Zika virus (ZIKV) and Japanese encephalitis virus (JEV) can cause permanent neurological damage and death, yet no approved drugs exist for these infections. Rhodiola crenulate, an herb used in traditional Chinese medicine for its antioxidation and antifatigue properties, was studied for its antiviral activity against ZIKV and JEV in vitro. The cytotoxicity of Rhodiola crenulata extract (RCE) was evaluated using the CCK-8 reagent. Antiviral effects of RCE were assessed in ZIKV-infected or JEV-infected Vero cells via quantitative reverse transcription polymerase chain reaction (qRT-PCR), Western blotting, fluorescent focus assay (FFA), and immunofluorescence assay (IFA). The cell-free antiviral effects of RCE were evaluated using an inactivation assay. To determine the stage of the viral life cycle affected by RCE, time-of-addition, binding, and entry assays were conducted. Three bioactive constituents of RCE (salidroside, tyrosol, and gallic acid) were tested for antiviral activity. RCE exhibited dose-dependent anti-ZIKV and anti-JEV activities at non-cytotoxic concentrations, which were likely achieved by disrupting viral binding and stability. Gallic acid exhibited antiviral activity against ZIKV and JEV. Our findings indicate that RCE disrupts viral binding and stability, presenting a potential strategy to treat ZIKV and JEV infections.
Keywords: Japanese encephalitis virus; Rhodiola crenulata; Zika virus; antiviral agent; gallic acid; salidroside.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Pielnaa P., Al-Saadawe M., Saro A., Dama M.F., Zhou M., Huang Y., Huang J., Xia Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020;543:34–42. doi: 10.1016/j.virol.2020.01.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

