Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis
- PMID: 39204094
- PMCID: PMC11357209
- DOI: 10.3390/ph17080989
Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis
Abstract
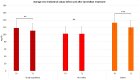
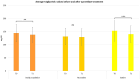
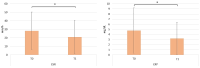
Psoriasis (PsO) is a chronic inflammatory dermatosis that often presents with erythematous, sharply demarcated lesions. Although psoriasis is primarily a dermatological disease, its immune-mediated pathogenesis produces systemic effects and is closely associated with various comorbid conditions such as cardiovascular disease (CVD), metabolic syndrome (MetS), and diabetes mellitus type II (DMII). Apremilast, an oral phosphodiesterase 4 (PDE-4) inhibitor, has shown promise in treating moderate-to-severe psoriasis and is associated with potential cardiometabolic benefits. In a 12-month prospective observational study involving 137 patients with moderate-to-severe psoriasis, we assessed changes in psoriasis clinimetric scores and metabolic profiles from baseline (T0) to 52 weeks (T1) to evaluate the efficacy of apremilast. After 52 weeks of apremilast treatment, we documented a statistically significant decrease in low-density lipoprotein (LDL) and total cholesterol, triglycerides, and glucose levels. Our findings even suggest a potential synergistic effect among patients treated with apremilast, alongside concomitant statin and/or insulin therapy. Although the results of our study must be validated on a larger scale, the use of apremilast in the treatment of psoriatic patients with cardio-metabolic comorbidities yields promising results.
Keywords: PDE4-inhibitor; apremilast; comorbidities; metabolic syndrome; psoriasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Kimmel G.W., Lebwohl M. Evidence-Based Psoriasis: Diagnosis and Treatment. Springer; Cham, Switzerland: 2018. Psoriasis: Overview and Diagnosis; pp. 1–16. - DOI
LinkOut - more resources
Full Text Sources

