Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia
- PMID: 39204095
- PMCID: PMC11356968
- DOI: 10.3390/ph17080990
Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia
Abstract
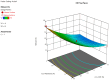
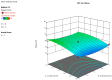
The intranasal route of drug administration is characterized by high bioavailability and is considered promising for rapid delivery of drugs with systemic action to the central nervous system (CNS), bypassing the blood-brain barrier. This is particularly important for the use of neuroprotective drugs in the treatment of brain tissue damage in infants caused by the effects of intrauterine hypoxia. The creation of new dosage forms for neonatology using mathematical technologies and special software in pharmaceutical development allows for the creation of cerebroprotective drugs with controlled pharmaco-technological properties, thus reducing time and resources for necessary research. We developed a new nasal gel formulation with Angiolin using a Box-Behnken experiment design for the therapy of prenatal CNS damage. It was found that the consistency characteristics of the nasal gel were significantly influenced by the gelling agent and mucoadhesive component-sodium salt of carboxymethylcellulose. We optimized the composition of nasal gel formulation with Angiolin using the formed models and relationships between the factors. The optimized nasal gel composition demonstrated satisfactory thixotropic properties. The 1% gel for neuroprotection with Angiolin, developed for intranasal administration, meets all safety requirements for this group of drug forms, showing low toxicity and no local irritant or allergic effects.
Keywords: angiolin; intranasal gel; neuroprotection; prenatal hypoxia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Formulation development and evaluation of nasal in situ gel of promethazine hydrochloride.Drug Dev Ind Pharm. 2024 Jan;50(1):11-22. doi: 10.1080/03639045.2023.2291463. Epub 2024 Jan 30. Drug Dev Ind Pharm. 2024. PMID: 38054848
-
Intranasal Zolmitriptan-Loaded Bilosomes with Extended Nasal Mucociliary Transit Time for Direct Nose to Brain Delivery.Pharmaceutics. 2021 Nov 1;13(11):1828. doi: 10.3390/pharmaceutics13111828. Pharmaceutics. 2021. PMID: 34834242 Free PMC article.
-
Development of in situ gel for nasal delivery: design, optimization, in vitro and in vivo evaluation.Drug Deliv. 2014 Feb;21(1):62-73. doi: 10.3109/10717544.2013.849778. Epub 2013 Nov 5. Drug Deliv. 2014. PMID: 24191774
-
Mucoadhesive in situ nasal gelling drug delivery systems for modulated drug delivery.Expert Opin Drug Deliv. 2013 Jan;10(1):115-30. doi: 10.1517/17425247.2013.746659. Epub 2012 Nov 30. Expert Opin Drug Deliv. 2013. PMID: 23199072 Review.
-
Convolutions in the rendition of nose to brain therapeutics from bench to bedside: Feats & fallacies.J Control Release. 2022 Jan;341:782-811. doi: 10.1016/j.jconrel.2021.12.009. Epub 2021 Dec 11. J Control Release. 2022. PMID: 34906605 Review.
Cited by
-
HSP70 Modulators for the Correction of Cognitive, Mnemonic, and Behavioral Disorders After Prenatal Hypoxia.Biomedicines. 2025 Apr 17;13(4):982. doi: 10.3390/biomedicines13040982. Biomedicines. 2025. PMID: 40299680 Free PMC article.
References
LinkOut - more resources
Full Text Sources

