Off-Label Use of Bevacizumab in Patients Diagnosed with Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis
- PMID: 39204105
- PMCID: PMC11357420
- DOI: 10.3390/ph17081000
Off-Label Use of Bevacizumab in Patients Diagnosed with Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis
Abstract
Background: Age-related macular degeneration (AMD) is the leading cause of vision loss in elderly people. Current pharmacological treatment in vascular AMD includes anti-VEGF agents, such as ranibizumab and aflibercept. Additionally, the off-label use of bevacizumab has been shown to be effective and has a lower cost, making it an interesting pharmacological approach; however, there is no consensus about its use. Therefore, this systematic review and meta-analysis aims to evaluate the efficacy, safety, and efficiency of bevacizumab in AMD patients.
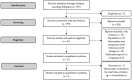
Methods: This review only focused on randomized controlled clinical trials published in 2010 in the MEDLINE database that compared the effect of bevacizumab with ranibizumab. The risk of bias in each included study was assessed using the CASP Randomised Clinical Trials checklist.
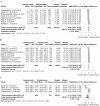
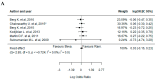
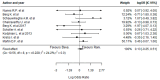
Results: Twelve studies were included for qualitative synthesis, and nine of them were considered for meta-analysis. Bevacizumab-treated patients showed a significantly reduced neovascularization in a longer spectrum of time; however, they had a higher incidence of endophthalmitis than those treated with ranibizumab. Regarding efficiency, the mean number of administrations was reduced in the treatment with bevacizumab in comparison to ranibizumab.
Conclusions: Clinical evidence demonstrates that bevacizumab has efficacy and safety profiles comparable with ranibizumab; however, it is relatively more efficient.
Keywords: AMD; age-related macular degeneration; bevacizumab; drug therapy; nAMD; neovascular age-related macular degeneration; wet macular degeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
The Efficacy, Safety, and Efficiency of the Off-Label Use of Bevacizumab in Patients Diagnosed With Age-Related Macular Degeneration: Protocol for a Systematic Review and Meta-Analysis.JMIR Res Protoc. 2023 Jun 9;12:e38658. doi: 10.2196/38658. JMIR Res Protoc. 2023. PMID: 37294608 Free PMC article.
-
Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2020 May 5;5(5):CD012208. doi: 10.1002/14651858.CD012208.pub2. Cochrane Database Syst Rev. 2020. PMID: 32374423 Free PMC article.
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2019 Mar 4;3(3):CD005139. doi: 10.1002/14651858.CD005139.pub4. Cochrane Database Syst Rev. 2019. PMID: 30834517 Free PMC article.
-
Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis.BMJ Open. 2019 May 28;9(5):e022031. doi: 10.1136/bmjopen-2018-022031. BMJ Open. 2019. PMID: 31142516 Free PMC article.
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials.BMC Ophthalmol. 2018 May 30;18(1):130. doi: 10.1186/s12886-018-0785-3. BMC Ophthalmol. 2018. PMID: 29843663 Free PMC article.
Cited by
-
Monoclonal Antibodies for the Treatment of Ocular Diseases.J Clin Med. 2024 Sep 28;13(19):5815. doi: 10.3390/jcm13195815. J Clin Med. 2024. PMID: 39407875 Free PMC article. Review.
-
Blue Light-Induced Mitochondrial Oxidative Damage Underlay Retinal Pigment Epithelial Cell Apoptosis.Int J Mol Sci. 2024 Nov 24;25(23):12619. doi: 10.3390/ijms252312619. Int J Mol Sci. 2024. PMID: 39684332 Free PMC article.
References
-
- Gil-Martínez M., Santos-Ramos P., Fernández-Rodríguez M., Abraldes M.J., Rodríguez-Cid M.J., Santiago-Varela M., Fernández-Ferreiro A., Gómez-Ulla F. Pharmacological Advances in the Treatment of Age-related Macular Degeneration. Curr. Med. Chem. 2020;27:583–598. doi: 10.2174/0929867326666190726121711. - DOI - PubMed
-
- Wong W., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

