Synthesis and Biochemical Evaluation of Ethanoanthracenes and Related Compounds: Antiproliferative and Pro-Apoptotic Effects in Chronic Lymphocytic Leukemia (CLL)
- PMID: 39204139
- PMCID: PMC11359702
- DOI: 10.3390/ph17081034
Synthesis and Biochemical Evaluation of Ethanoanthracenes and Related Compounds: Antiproliferative and Pro-Apoptotic Effects in Chronic Lymphocytic Leukemia (CLL)
Abstract
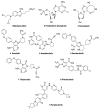
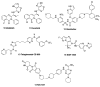
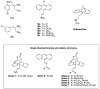
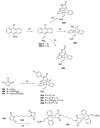
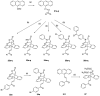
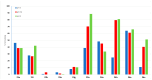
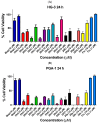
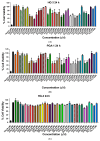
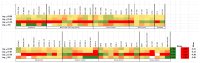
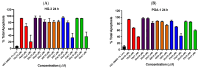
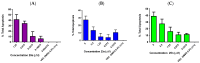
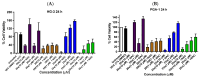
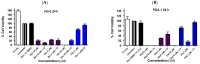
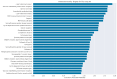
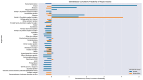
Chronic lymphocytic leukemia (CLL) is a malignancy of mature B cells, and it is the most frequent form of leukemia diagnosed in Western countries. It is characterized by the proliferation and accumulation of neoplastic B lymphocytes in the blood, lymph nodes, bone marrow and spleen. We report the synthesis and antiproliferative effects of a series of novel ethanoanthracene compounds in CLL cell lines. Structural modifications were achieved via the Diels-Alder reaction of 9-(2-nitrovinyl)anthracene and 3-(anthracen-9-yl)-1-arylprop-2-en-1-ones (anthracene chalcones) with dienophiles, including maleic anhydride and N-substituted maleimides, to afford a series of 9-(E)-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones, 9-(E)-3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones and related compounds. Single-crystal X-ray analysis confirmed the structures of the novel ethanoanthracenes 23f, 23h, 24a, 24g, 25f and 27. The products were evaluated in HG-3 and PGA-1 CLL cell lines (representative of poor and good patient prognosis, respectively). The most potent compounds were identified as 20a, 20f, 23a and 25n with IC50 values in the ranges of 0.17-2.69 µM (HG-3) and 0.35-1.97 µM (PGA-1). The pro-apoptotic effects of the potent compounds 20a, 20f, 23a and 25n were demonstrated in CLL cell lines HG-3 (82-95%) and PGA-1 (87-97%) at 10 µM, with low toxicity (12-16%) observed in healthy-donor peripheral blood mononuclear cells (PBMCs) at concentrations representative of the compounds IC50 values for both the HG-3 and PGA-1 CLL cell lines. The antiproliferative effect of the selected compounds, 20a, 20f, 23a and 25n, was mediated through ROS flux with a marked increase in cell viability upon pretreatment with the antioxidant NAC. 25n also demonstrated sub-micromolar activity in the NCI 60 cancer cell line panel, with a mean GI50 value of 0.245 µM. This ethanoanthracene series of compounds offers potential for the further development of lead structures as novel chemotherapeutics to target CLL.
Keywords: B cell; antiproliferative; chalcone; chronic lymphocytic leukemia (CLL); ethanoanthracene; pro-apoptotic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Synthesis and Pro-Apoptotic Effects of Nitrovinylanthracenes and Related Compounds in Chronic Lymphocytic Leukaemia (CLL) and Burkitt's Lymphoma (BL).Molecules. 2023 Dec 14;28(24):8095. doi: 10.3390/molecules28248095. Molecules. 2023. PMID: 38138584 Free PMC article.
-
Design, Synthesis and Biochemical Evaluation of Novel Ethanoanthracenes and Related Compounds to Target Burkitt's Lymphoma.Pharmaceuticals (Basel). 2020 Jan 17;13(1):16. doi: 10.3390/ph13010016. Pharmaceuticals (Basel). 2020. PMID: 31963567 Free PMC article.
-
Synthesis and antiproliferative action of a novel series of maprotiline analogues.Eur J Med Chem. 2014 Jan;71:333-53. doi: 10.1016/j.ejmech.2013.10.076. Epub 2013 Nov 9. Eur J Med Chem. 2014. PMID: 24333581
-
Survival and Immunosuppression Induced by Hepatocyte Growth Factor in Chronic Lymphocytic Leukemia.Curr Mol Med. 2017;17(1):24-33. doi: 10.2174/1566524017666170220095838. Curr Mol Med. 2017. PMID: 28231754 Review.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
References
-
- Cancer Stat Facts: Leukemia—Chronic Lymphocytic Leukemia (CLL) National Cancer Institute. [(accessed on 26 April 2024)];2022 Available online: https://seer.cancer.gov/statfacts/html/clyl.html.
-
- Waldron C., O’Brien D., Brophy S., Perera K., Crotty G.M., Dunlea E., Walsh A., Connolly M., Clifford R., O’Leary H., et al. Epidemiology of chronic lymphocytic leukaemia in an Irish subpopulation with total case ascertainment: An additional tool for health economic planning. Br. J. Haematol. 2022;196:e47–e49. doi: 10.1111/bjh.17929. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

