Gefitinib-Induced Severe Dermatological Adverse Reactions: A Case Report and Pharmacogenetic Profile
- PMID: 39204145
- PMCID: PMC11359302
- DOI: 10.3390/ph17081040
Gefitinib-Induced Severe Dermatological Adverse Reactions: A Case Report and Pharmacogenetic Profile
Abstract
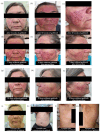
Gefitinib is a selective inhibitor of the epidermal growth factor receptor that is used to treat advanced and metastatic non-small cell lung cancer (NSCLC). Dermatological adverse reactions are most commonly associated with gefitinib treatment. The cause of adverse reactions in individuals is multifactorial. Pharmacogenetics is an effective tool to detect such adverse reactions. This case report describes a female patient with NSCLC who was administered gefitinib at a dose of 250 mg/day. However, due to severe adverse dermatological reactions, the treatment was interrupted for 15 d and antibiotic therapy was administered to manage the skin rashes, maculopapular rashes, and hyperpigmentation. Treatment adherence was adequate, and no drug interactions were detected. A pharmacogenetic analysis revealed homozygosity in the ATP-binding cassette (ABC)-B1 rs1128503 (c.1236A>G), heterozygosity in ABCG2 rs2231142 (c.421G>T) and rs2622604 (c.-20+614T>C), and a non-functional variant of the cytochrome P450 family 3, subfamily A, member 5 (CYP3A5). The relationship between altered genetic variants and the presence of adverse reactions induced by gefitinib is still controversial. Overall, this case report highlights the importance of continuing to study pharmacogenetics as predictors of adverse drug reactions.
Keywords: ABCB1; adverse drug reaction; case report; cytochrome P450 enzyme system; gefitinib; pharmacogenetics; skin rash.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Pharmacogenetics of ABCG2 and adverse reactions to gefitinib.J Natl Cancer Inst. 2006 Dec 6;98(23):1739-42. doi: 10.1093/jnci/djj469. J Natl Cancer Inst. 2006. PMID: 17148776
-
Effect of genetic polymorphisms on the pharmacokinetics of gefitinib in healthy Chinese volunteers.Xenobiotica. 2024 Jan;54(1):38-44. doi: 10.1080/00498254.2023.2294039. Epub 2023 Dec 18. Xenobiotica. 2024. PMID: 38085693
-
Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity.Nagoya J Med Sci. 2012 Feb;74(1-2):133-40. Nagoya J Med Sci. 2012. PMID: 22515119 Free PMC article.
-
The Pharmacogenetic Rescue of Side-Lined Anticancer Drugs to the Front-Line: Gefitinib as a Case Example.Ann Pharmacother. 2011 Feb;45(2):263-75. doi: 10.1345/aph.1P496. Ann Pharmacother. 2011. PMID: 21304034 Review.
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD010383. doi: 10.1002/14651858.CD010383.pub3. Cochrane Database Syst Rev. 2021. PMID: 33734432 Free PMC article.
References
-
- Cohen M.H., Williams G.A., Sridhara R., Chen G., McGuinn W.D., Morse D., Abraham S., Rahman A., Liang C., Lostritto R., et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin. Cancer Res. 2004;10:1212–1218. doi: 10.1158/1078-0432.CCR-03-0564. - DOI - PubMed
-
- Hofheinz R.-D., Deplanque G., Komatsu Y., Kobayashi Y., Ocvirk J., Racca P., Guenther S., Zhang J., Lacouture M.E., Jatoi A. Recommendations for the Prophylactic Management of Skin Reactions Induced by Epidermal Growth Factor Receptor Inhibitors in Patients with Solid Tumors. Oncologist. 2016;21:1483–1491. doi: 10.1634/theoncologist.2016-0051. - DOI - PMC - PubMed
Publication types
Grants and funding
- 14030/2019-4/National Council for Scientific and Technological Development
- 2023/06280-7/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 444090/2023/National Council for Scientific and Technological Development
- no.895688/2019/Ministry of Justice and Public Security of Brazil, through the Fund for the Defense of Diffuse Rights
LinkOut - more resources
Full Text Sources
Research Materials

