Real-World Application of a Quantitative Systems Pharmacology (QSP) Model to Predict Potassium Concentrations from Electronic Health Records: A Pilot Case towards Prescribing Monitoring of Spironolactone
- PMID: 39204148
- PMCID: PMC11357243
- DOI: 10.3390/ph17081041
Real-World Application of a Quantitative Systems Pharmacology (QSP) Model to Predict Potassium Concentrations from Electronic Health Records: A Pilot Case towards Prescribing Monitoring of Spironolactone
Abstract
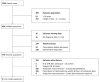
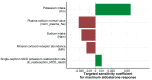
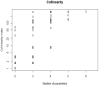
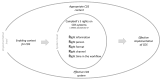
Quantitative systems pharmacology (QSP) models are rarely applied prospectively for decision-making in clinical practice. We therefore aimed to operationalize a QSP model for potas-sium homeostasis to predict potassium trajectories based on spironolactone administrations. For this purpose, we proposed a general workflow that was applied to electronic health records (EHR) from patients treated in a German tertiary care hospital. The workflow steps included model exploration, local and global sensitivity analyses (SA), identifiability analysis (IA) of model parameters, and specification of their inter-individual variability (IIV). Patient covariates, selected parameters, and IIV then defined prior information for the Bayesian a posteriori prediction of individual potassium trajectories of the following day. Following these steps, the successfully operationalized QSP model was interactively explored via a Shiny app. SA and IA yielded five influential and estimable parameters (extracellular fluid volume, hyperaldosteronism, mineral corticoid receptor abundance, potassium intake, sodium intake) for Bayesian prediction. The operationalized model was validated in nine pilot patients and showed satisfactory performance based on the (absolute) average fold error. This provides proof-of-principle for a Prescribing Monitoring of potassium concentrations in a hospital system, which could suggest preemptive clinical measures and therefore potentially avoid dangerous hyperkalemia or hypokalemia.
Keywords: electronic health records (EHR); kidney; maximum a posteriori (MAP) (Bayesian) estimation; potassium; quantitative systems pharmacology (QSP); sensitivity analysis; spironolactone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Translational Quantitative Systems Pharmacology in Drug Development: from Current Landscape to Good Practices.AAPS J. 2019 Jun 3;21(4):72. doi: 10.1208/s12248-019-0339-5. AAPS J. 2019. PMID: 31161268 Review.
-
A quantitative systems pharmacology model of plasma potassium regulation by the kidney and aldosterone.J Pharmacokinet Pharmacodyn. 2022 Aug;49(4):471-486. doi: 10.1007/s10928-022-09815-x. Epub 2022 Jul 1. J Pharmacokinet Pharmacodyn. 2022. PMID: 35776281
-
Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist.Circ Heart Fail. 2014 Jul;7(4):573-9. doi: 10.1161/CIRCHEARTFAILURE.114.001104. Epub 2014 May 8. Circ Heart Fail. 2014. PMID: 24812304 Clinical Trial.
-
FDA-Industry Scientific Exchange on assessing quantitative systems pharmacology models in clinical drug development: a meeting report, summary of challenges/gaps, and future perspective.AAPS J. 2021 Apr 30;23(3):60. doi: 10.1208/s12248-021-00585-x. AAPS J. 2021. PMID: 33931790
-
A Review of Quantitative Systems Pharmacology Models of the Coagulation Cascade: Opportunities for Improved Usability.Pharmaceutics. 2023 Mar 11;15(3):918. doi: 10.3390/pharmaceutics15030918. Pharmaceutics. 2023. PMID: 36986779 Free PMC article. Review.
References
-
- Helmlinger G., Sokolov V., Peskov K., Hallow K.M., Kosinsky Y., Voronova V., Chu L., Yakovleva T., Azarov I., Kaschek D., et al. Quantitative Systems Pharmacology: An Exemplar Model-Building Workflow with Applications in Cardiovascular, Metabolic, and Oncology Drug Development. CPT Pharmacomet. Syst. Pharmacol. 2019;8:380–395. doi: 10.1002/psp4.12426. - DOI - PMC - PubMed
-
- Lasiter L., Tymejczyk O., Garrett-Mayer E., Baxi S., Belli A.J., Boyd M., Christian J.B., Cohen A.B., Espirito J.L., Hansen E., et al. Real-world Overall Survival Using Oncology Electronic Health Record Data: Friends of Cancer Research Pilot. Clin. Pharmacol. Ther. 2022;111:444–454. doi: 10.1002/cpt.2443. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

