Real-World Application of a Quantitative Systems Pharmacology (QSP) Model to Predict Potassium Concentrations from Electronic Health Records: A Pilot Case towards Prescribing Monitoring of Spironolactone
- PMID: 39204148
- PMCID: PMC11357243
- DOI: 10.3390/ph17081041
Real-World Application of a Quantitative Systems Pharmacology (QSP) Model to Predict Potassium Concentrations from Electronic Health Records: A Pilot Case towards Prescribing Monitoring of Spironolactone
Abstract
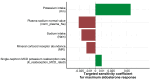
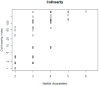
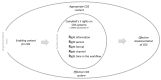
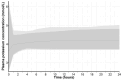
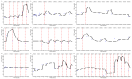
Quantitative systems pharmacology (QSP) models are rarely applied prospectively for decision-making in clinical practice. We therefore aimed to operationalize a QSP model for potas-sium homeostasis to predict potassium trajectories based on spironolactone administrations. For this purpose, we proposed a general workflow that was applied to electronic health records (EHR) from patients treated in a German tertiary care hospital. The workflow steps included model exploration, local and global sensitivity analyses (SA), identifiability analysis (IA) of model parameters, and specification of their inter-individual variability (IIV). Patient covariates, selected parameters, and IIV then defined prior information for the Bayesian a posteriori prediction of individual potassium trajectories of the following day. Following these steps, the successfully operationalized QSP model was interactively explored via a Shiny app. SA and IA yielded five influential and estimable parameters (extracellular fluid volume, hyperaldosteronism, mineral corticoid receptor abundance, potassium intake, sodium intake) for Bayesian prediction. The operationalized model was validated in nine pilot patients and showed satisfactory performance based on the (absolute) average fold error. This provides proof-of-principle for a Prescribing Monitoring of potassium concentrations in a hospital system, which could suggest preemptive clinical measures and therefore potentially avoid dangerous hyperkalemia or hypokalemia.
Keywords: electronic health records (EHR); kidney; maximum a posteriori (MAP) (Bayesian) estimation; potassium; quantitative systems pharmacology (QSP); sensitivity analysis; spironolactone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Helmlinger G., Sokolov V., Peskov K., Hallow K.M., Kosinsky Y., Voronova V., Chu L., Yakovleva T., Azarov I., Kaschek D., et al. Quantitative Systems Pharmacology: An Exemplar Model-Building Workflow with Applications in Cardiovascular, Metabolic, and Oncology Drug Development. CPT Pharmacomet. Syst. Pharmacol. 2019;8:380–395. doi: 10.1002/psp4.12426. - DOI - PMC - PubMed
-
- Lasiter L., Tymejczyk O., Garrett-Mayer E., Baxi S., Belli A.J., Boyd M., Christian J.B., Cohen A.B., Espirito J.L., Hansen E., et al. Real-world Overall Survival Using Oncology Electronic Health Record Data: Friends of Cancer Research Pilot. Clin. Pharmacol. Ther. 2022;111:444–454. doi: 10.1002/cpt.2443. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

