Characterisation of European Field Goat Prion Isolates in Ovine PrP Overexpressing Transgenic Mice (Tgshp IX) Reveals Distinct Prion Strains
- PMID: 39204230
- PMCID: PMC11357236
- DOI: 10.3390/pathogens13080629
Characterisation of European Field Goat Prion Isolates in Ovine PrP Overexpressing Transgenic Mice (Tgshp IX) Reveals Distinct Prion Strains
Abstract
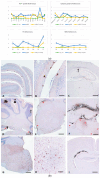
After the detection of bovine spongiform encephalopathy (BSE), and a zoonotic transmissible spongiform encephalopathy (TSE) caused by the pathological prion protein (PrPSc) in two goats, the investigation of goat prions became of greater interest. Therefore, a broad collection of European goat TSE isolates, including atypical scrapie, CH1641 and goat BSE as reference prion strains were biochemically characterised and subsequently inoculated into seven rodent models for further analysis (already published results of this comprehensive study are reviewed here for comparative reasons). We report here the histopathological and immunohistochemical data of this goat TSE panel, obtained after the first passage in Tgshp IX (tg-shARQ) mice, which overexpress the ovine prion protein. In addition to the clear-cut discrimination of all reference prion strains from the classical scrapie (CS) isolates, we were further able to determine three categories of CS strains. The investigation further indicates the occurrence of sub-strains that slightly resemble distant TSE strains, such as BSE or CH1641, reinforcing the theory that CS is not a single strain but a mixture of sub-strains, existing at varying extents in one isolate. This study further proved that Tgshp IX is a potent and reliable tool for the in-depth characterisation of prion strains.
Keywords: BSE; CH1641-like; PrPSc; Tgshp IX; atypical scrapie; bovine spongiform encephalopathy; classical scrapie; goat; immunohistochemistry; strain typing; transgenic mice.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

