Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health
- PMID: 39204297
- PMCID: PMC11356977
- DOI: 10.3390/pathogens13080697
Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health
Abstract
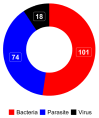
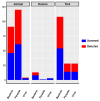
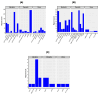
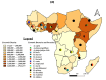
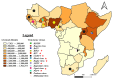
Sub-Saharan Africa, with its hot and humid climate, is a conducive zone for tick proliferation. These vectors pose a major challenge to both animal and human health in the region. However, despite the relevance of emerging diseases and evidence of tick-borne disease emergence, very few studies have been dedicated to investigating zoonotic pathogens transmitted by ticks in this area. To raise awareness of the risks of tick-borne zoonotic diseases in sub-Saharan Africa, and to define a direction for future research, this systematic review considers the trends of research on tick-borne bacteria, parasites, and viruses from 2012 to 2023, aiming to highlight the circulation of these pathogens in ticks, cattle, sheep, goats, and humans. For this purpose, three international databases were screened to select 159 papers fitting designed inclusion criteria and used for qualitative analyses. Analysis of these studies revealed a high diversity of tick-borne pathogens in sub-Saharan Africa, with a total of 37 bacterial species, 27 parasite species, and 14 viruses identified. Among these, 27% were zoonotic pathogens, yet only 11 studies investigated their presence in humans. Furthermore, there is growing interest in the investigation of bacteria and parasites in both ticks and ruminants. However, research into viruses is limited and has only received notable interest from 2021 onwards. While studies on the detection of bacteria, including those of medical interest, have focused on ticks, little consideration has been given to these vectors in studies of parasites circulation. Regarding the limited focus on zoonotic pathogens transmitted by ticks, particularly in humans, despite documented cases of emerging zoonoses and the notable 27% proportion reported, further efforts should be made to fill these gaps. Future studies should prioritize the investigation of zoonotic pathogens, especially viruses, which represent the primary emerging threats, by adopting a One Health approach. This will enhance the understanding of their circulation and impact on both human and animal health. In addition, more attention should be given to the risk factors/drivers associated to their emergence as well as the perception of the population at risk of infection from these zoonotic pathogens.
Keywords: pathogens; public health; research; sub-Sahara Africa; systematic review; tick-borne diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Muhammad A., Piyumali K.P., Abdullah I., Shumaila M. Ticks and Tick-Borne pathogens. Volume 9. IntechOpen.; London, UK: 2018. Ticks and Tick-Borne Pathogens; pp. 3–9.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

