Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum
- PMID: 39204328
- PMCID: PMC11357599
- DOI: 10.3390/pharmaceutics16080983
Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum
Erratum in
-
Correction: Elalouf et al. Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum. Pharmaceutics 2024, 16, 983.Pharmaceutics. 2024 Sep 29;16(10):1273. doi: 10.3390/pharmaceutics16101273. Pharmaceutics. 2024. PMID: 39458675 Free PMC article.
Abstract
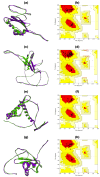
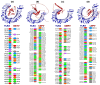
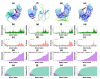
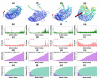
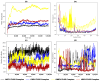
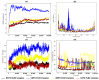
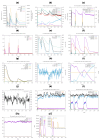
Tinea cruris, a dermatophyte fungal infection predominantly caused by Trichophyton rubrum and Epidermophyton floccosum, primarily affects the groin, pubic region, and adjacent thigh. Its recurrence is frequent, attributable to repeated fungal infections in susceptible individuals, especially those with onychomycosis or tinea pedis, which act as reservoirs for dermatophytes. Given the persistent nature of tinea cruris, vaccination emerges as a promising strategy for fungal infection management, offering targeted, durable protection against various fungal species. Vaccines stimulate both humoral and cell-mediated immunity and are administered prophylactically to prevent infections while minimizing the risk of antifungal resistance development. Developing fungal vaccines is challenging due to the thick fungal cell wall, similarities between fungal and human cells, antigenic variation, and evolutionary resemblance to animals, complicating non-toxic target identification and T-cell response variability. No prior research has shown an mRNA vaccine for T. rubrum. Hence, this study proposes a novel mRNA-based vaccine for tinea cruris, potentially offering long-term immunity and reducing reliance on antifungal medications. This study explores the complete proteome of T. rubrum, identifying potential protein candidates for vaccine development through reverse vaccinology. Immunogenic epitopes from these candidates were mapped and integrated into multitope vaccines and reverse translated to construct mRNA vaccines. Then, the mRNA was translated and computationally assessed for physicochemical, chemical, and immunological attributes. Notably, 1,3-beta-glucanosyltransferase, CFEM domain-containing protein, cell wall galactomannoprotein, and LysM domain-containing protein emerged as promising vaccine targets. Antigenic, immunogenic, non-toxic, and non-allergenic cytotoxic T lymphocyte, helper T lymphocyte, and B lymphocyte epitopes were selected and linked with appropriate linkers and Toll-like receptor (TLR) agonist adjuvants to formulate vaccine candidates targeting T. rubrum. The protein-based vaccines underwent reverse translation to construct the mRNA vaccines, which, after inoculation, were translated again by host ribosomes to work as potential components for triggering the immune response. After that, molecular docking, normal mode analysis, and molecular dynamic simulation confirmed strong binding affinities and stable complexes between vaccines and TLR receptors. Furthermore, immune simulations of vaccines with and without adjuvant demonstrated activation of immune responses, evidenced by elevated levels of IgG1, IgG2, IgM antibodies, cytokines, and interleukins. There was no significant change in antibody production between vaccines with and without adjuvants, but adjuvants are crucial for activating the innate immune response via TLRs. Although mRNA vaccines hold promise against fungal infections, further research is essential to assess their safety and efficacy. Experimental validation is crucial for evaluating their immunogenicity, effectiveness, and safety.
Keywords: Trichophyton rubrum; bioinformatics; mRNA-based vaccine; tinea cruris.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Design of a multi-epitope vaccine against six Nocardia species based on reverse vaccinology combined with immunoinformatics.Front Immunol. 2023 Feb 2;14:1100188. doi: 10.3389/fimmu.2023.1100188. eCollection 2023. Front Immunol. 2023. PMID: 36845087 Free PMC article.
-
An integrated multi-pronged reverse vaccinology and biophysical approaches for identification of potential vaccine candidates against Nipah virus.Saudi Pharm J. 2023 Dec;31(12):101826. doi: 10.1016/j.jsps.2023.101826. Epub 2023 Oct 16. Saudi Pharm J. 2023. PMID: 38028215 Free PMC article.
-
The Immunologic Response to Trichophyton Rubrum in Lower Extremity Fungal Infections.J Fungi (Basel). 2015 Jul 17;1(2):130-137. doi: 10.3390/jof1020130. J Fungi (Basel). 2015. PMID: 29376904 Free PMC article. Review.
-
Exploring whole proteome to contrive multi-epitope-based vaccine for NeoCoV: An immunoinformtics and in-silico approach.Front Immunol. 2022 Aug 3;13:956776. doi: 10.3389/fimmu.2022.956776. eCollection 2022. Front Immunol. 2022. PMID: 35990651 Free PMC article.
-
Reverse engineering protection: A comprehensive survey of reverse vaccinology-based vaccines targeting viral pathogens.Vaccine. 2024 Apr 11;42(10):2503-2518. doi: 10.1016/j.vaccine.2024.02.087. Epub 2024 Mar 23. Vaccine. 2024. PMID: 38523003 Review.
Cited by
-
Correction: Elalouf et al. Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum. Pharmaceutics 2024, 16, 983.Pharmaceutics. 2024 Sep 29;16(10):1273. doi: 10.3390/pharmaceutics16101273. Pharmaceutics. 2024. PMID: 39458675 Free PMC article.
-
Synthesis of an Adjuvant-Free Single Polypeptide-Based Tuberculosis Subunit Vaccine that Elicits In Vivo Immunogenicity in Rats.Mol Biotechnol. 2025 Apr 2. doi: 10.1007/s12033-025-01431-7. Online ahead of print. Mol Biotechnol. 2025. PMID: 40175786
References
-
- Bishnoi A., Mahajan R. Diagnostics to Pathogenomics of Sexually Transmitted Infections. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2023. Tinea Cruris; pp. 329–340. - DOI
-
- Ismail F., Ghani A., Akbar S. Emergence of Antifungal Azole Resistance in the Fungal Strains of Tinea Corporis, Tinea Capitis, Tinea Cruris and Tinea Pedis from the Locality of Southern Punjab, Pakistan. RADS J. Biol. Res. Appl. Sci. 2021;12:24–38. doi: 10.37962/jbas.v12i1.348. - DOI
-
- Kashif S., Uddin F., Nasir F., Zafar S., Shahjabee, Kumar S. Prevalence of Dermatophytes in Superficial Skin Infections in a Tertiary Care Hospital. J. Pak. Assoc. Dermatol. 2021;31:484–488.
LinkOut - more resources
Full Text Sources

