In Vitro Studies to Evaluate the Intestinal Permeation of an Ursodeoxycholic Acid-Conjugated Oligonucleotide for Duchenne Muscular Dystrophy Treatment
- PMID: 39204368
- PMCID: PMC11360444
- DOI: 10.3390/pharmaceutics16081023
In Vitro Studies to Evaluate the Intestinal Permeation of an Ursodeoxycholic Acid-Conjugated Oligonucleotide for Duchenne Muscular Dystrophy Treatment
Abstract
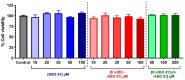
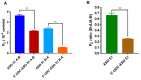
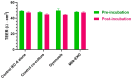
Delivery represents a major hurdle to the clinical advancement of oligonucleotide therapeutics for the treatment of disorders such as Duchenne muscular dystrophy (DMD). In this preliminary study, we explored the ability of 2'-O-methyl-phosphorothioate antisense oligonucleotides (ASOs) conjugated with lipophilic ursodeoxycholic acid (UDCA) to permeate across intestinal barriers in vitro by a co-culture system of non-contacting IEC-6 cells and DMD myotubes, either alone or encapsulated in exosomes. UDCA was used to enhance the lipophilicity and membrane permeability of ASOs, potentially improving oral bioavailability. Exosomes were employed due to their biocompatibility and ability to deliver therapeutic cargo across biological barriers. Exon skipping was evaluated in the DMD myotubes to reveal the targeting efficiency. Exosomes extracted from milk and wild-type myotubes loaded with 5'-UDC-3'Cy3-ASO and seeded directly on DMD myotubes appear able to fuse to myotubes and induce exon skipping, up to ~20%. Permeation studies using the co-culture system were performed with 5'-UDC-3'Cy3-ASO 51 alone or loaded in milk-derived exosomes. In this setting, only gymnotic delivery induced significant levels of exon skipping (almost 30%) implying a possible role of the intestinal cells in enhancing delivery of ASOs. These results warrant further investigations to elucidate the delivery of ASOs by gymnosis or exosomes.
Keywords: DMD myotubes; Duchenne muscular dystrophy; IEC-6 cells; antisense oligonucleotides; co-culture; conjugated oligonucleotides; exon skipping; exosomes; intestinal permeation; ursodeoxycholic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- FDA Approves Nonsteroidal Treatment for Duchenne Muscular Dystrophy. [(accessed on 27 June 2024)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-nonster....
Grants and funding
LinkOut - more resources
Full Text Sources

