Identification of the Biotransformation Pathways of a Potential Oral Male Contraceptive, 11β-Methyl-19-Nortestosterone (11β-MNT) and Its Prodrugs: An In Vitro Study Highlights the Contribution of Polymorphic Intestinal UGT2B17
- PMID: 39204377
- PMCID: PMC11360557
- DOI: 10.3390/pharmaceutics16081032
Identification of the Biotransformation Pathways of a Potential Oral Male Contraceptive, 11β-Methyl-19-Nortestosterone (11β-MNT) and Its Prodrugs: An In Vitro Study Highlights the Contribution of Polymorphic Intestinal UGT2B17
Abstract
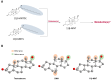
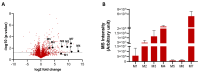
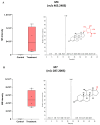
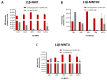
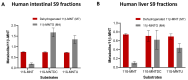
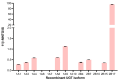
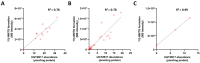
11β-Methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC) is a prodrug of 11β-MNT and is being considered as a promising male oral contraceptive candidate in clinical development. However, the oral administration of 11β-MNTDC exhibits an ~200-fold lower serum concentration of 11β-MNT compared to 11β-MNTDC, resulting in the poor bioavailability of 11β-MNT. To elucidate the role of the first-pass metabolism of 11β-MNT in its poor bioavailability, we determined the biotransformation products of 11β-MNT and its prodrugs in human in vitro models. 11β-MNT and its two prodrugs 11β-MNTDC and 11β-MNT undecanoate (11β-MNTU) were incubated in cryopreserved human hepatocytes (HHs) and subjected to liquid chromatography-high resolution tandem mass spectrometry analysis, which identified ten 11β-MNT biotransformation products with dehydrogenated and glucuronidation (11β-MNTG) metabolites being the major metabolites. However, 11β-MNTG formation is highly variable and prevalent in human intestinal S9 fractions. A reaction phenotyping study of 11β-MNT using thirteen recombinant UDP-glucuronosyltransferase (UGT) enzymes confirmed the major role of UGT2B17 in 11β-MNTG formation. This was further supported by a strong correlation (R2 > 0.78) between 11β-MNTG and UGT2B17 abundance in human intestinal microsomes, human liver microsomes, and HH systems. These results suggest that 11β-MNT and its prodrugs are rapidly metabolized to 11β-MNTG by the highly polymorphic intestinal UGT2B17, which may explain the poor and variable bioavailability of the drug.
Keywords: 11β-MNT; 11β-MNTDC; 11β-Methyl-19-nortestosterone; LC-MS; MetID; UGT2B17; biotransformation; metabolite identification; oral male contraceptive.
Conflict of interest statement
Bhagwat Prasad (Corresponding author) is a co-founder of Precision Quantomics Inc. and a recipient of research funding from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Generation Bio, Gilead, Merck, Novartis, and Takeda. All other authors declare no conflicts of interest.
Figures
References
-
- Attardi B.J., Hild S.A., Koduri S., Pham T., Pessaint L., Engbring J., Till B., Gropp D., Semon A., Reel J.R. The Potent Synthetic Androgens, Dimethandrolone (7α,11β-Dimethyl-19-nortestosterone) and 11β-Methyl-19-nortestosterone, Do Not Require 5α-Reduction to Exert Their Maximal Androgenic Effects. J. Steroid Biochem. Mol. Biol. 2010;122:212–218. doi: 10.1016/j.jsbmb.2010.06.009. - DOI - PMC - PubMed
-
- Sharma S., Ahire D., Basit A., Lajoie M., Wang C., Lee M.S., Blithe D.L., Amory J.K., Singh D.K., Heyward S., et al. Dimethandrolone, a Potential Male Contraceptive Pill, Is Primarily Metabolized by the Highly Polymorphic UDP-Glucuronosyltransferase 2B17 Enzyme in Human Intestine and Liver. Drug Metab. Dispos. 2022;50:1493–1500. doi: 10.1124/dmd.122.001041. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

