Polyene-Based Derivatives with Antifungal Activities
- PMID: 39204411
- PMCID: PMC11360744
- DOI: 10.3390/pharmaceutics16081065
Polyene-Based Derivatives with Antifungal Activities
Abstract
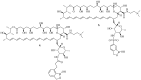
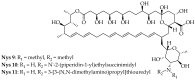
Polyenes are a class of organic compounds well known for their potent antifungal properties. They are effective due to their ability to target and disrupt fungal cell membranes by binding to ergosterol and forming pores. Despite their effectiveness as antifungal drugs, polyenes have several limitations, such as high toxicity to the host cell and poor solubility in water. This has prompted ongoing research to develop safer and more efficient derivatives to overcome such limitations while enhancing their antifungal activity. In this review article, we present a thorough analysis of polyene derivatives, their structural modifications, and their influence on their therapeutic effects against various fungal strains. Key studies are discussed, illustrating how structural modifications have led to improved antifungal properties. By evaluating the latest advancements in the synthesis of polyene derivatives, we highlight that incorporating amide linkers at the carboxylic moiety of polyene molecules notably improves their antifungal properties, as evidenced by derivatives 4, 5, 6G, and 18. This review can help in the design and development of novel polyene-based compounds with potent antifungal activities.
Keywords: antifungal activity; hybrid compounds; polyene-based derivatives; polyenes; potency.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

