Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential
- PMID: 39204547
- PMCID: PMC11359050
- DOI: 10.3390/polym16162327
Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential
Abstract
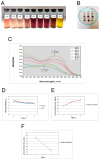
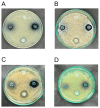
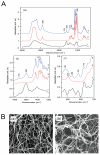
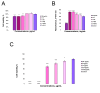
Polymer-based dressings deriving from natural biomaterials have advantages such as nontoxicity, biocompatibility, and mechanical stability, which are essential for efficient wound healing and microbial infection diagnostics. Here, we designed a prototype of an intelligent hydrogel dressing on the base of bacterial cellulose (BC) for monitoring wound microbial infection due to the uploaded natural pH dye-sensor, anthocyanins (ANC) of elderberry fruit (Sambucus nigra L.). The highest sensor responses to bacterial metabolites for ANC immobilized to BC were observed at pH 5.0 and 6.0. The detection limit of the sensor signals was 3.45 A.U., as it was evaluated with a smartphone-installed application. The FTIR spectral analysis of the hybrid BC/ANC hydrogel films has proved the presence of anthocyanins within the BC matrix. Hybrid films differed from the control ones by thicker microfibrils and larger pores, as detected with scanning electron microscopy. Halochromic BC/ANC films exhibited antimicrobial activities mainly against gram-positive bacteria and yeast. They showed no cytotoxicity for the in vitro human cell lines and mouse fibroblasts within a selected range of anthocyanin concentrations released from the BC/ANC film/dressing prototype. Compared to the control, the in vitro healing test showed overgrowth of primary mouse fibroblasts after applying 0.024-2.4 µg/mL ANC.
Keywords: anthocyanins; bacterial cellulose; hybrid polymer; microbial infection; natural pH-sensor; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dou L., Bai Y., Liu M., Shao S., Yang H., Yu X., Wen K., Wang Z., Shen J., Yu W. ‘Three-To-One’ Multi-Functional Nanocomposite-Based Lateral Flow Immunoassay for Label-Free and Dual-Readout Detection of Pathogenic Bacteria. Biosens. Bioelectron. 2022;204:114093. doi: 10.1016/j.bios.2022.114093. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

