Design and Optimization of Molecularly Imprinted Polymer Targeting Epinephrine Molecule: A Theoretical Approach
- PMID: 39204561
- PMCID: PMC11359759
- DOI: 10.3390/polym16162341
Design and Optimization of Molecularly Imprinted Polymer Targeting Epinephrine Molecule: A Theoretical Approach
Abstract
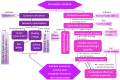
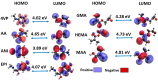
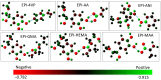
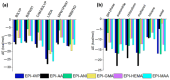
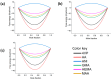
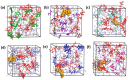
Molecularly imprinted polymers (MIPs) are a growing highlight in polymer chemistry. They are chemically and thermally stable, may be used in a variety of environments, and fulfill a wide range of applications. Computer-aided studies of MIPs often involve the use of computational techniques to design, analyze, and optimize the production of MIPs. Limited information is available on the computational study of interactions between the epinephrine (EPI) MIP and its target molecule. A rational design for EPI-MIP preparation was performed in this study. First, density functional theory (DFT) and molecular dynamic (MD) simulation were used for the screening of functional monomers suitable for the design of MIPs of EPI in the presence of a crosslinker and a solvent environment. Among the tested functional monomers, acrylic acid (AA) was the most appropriate monomer for EPI-MIP formulation. The trends observed for five out of six DFT functionals assessed confirmed AA as the suitable monomer. The theoretical optimal molar ratio was 1:4 EPI:AA in the presence of ethylene glycol dimethacrylate (EGDMA) and acetonitrile. The effect of temperature was analyzed at this ratio of EPI:AA on mean square displacement, X-ray diffraction, density distribution, specific volume, radius of gyration, and equilibrium energies. The stability observed for all these parameters is much better, ranging from 338 to 353 K. This temperature may determine the processing and operating temperature range of EPI-MIP development using AA as a functional monomer. For cost-effectiveness and to reduce time used to prepare MIPs in the laboratory, these results could serve as a useful template for designing and developing EPI-MIPs.
Keywords: epinephrine; molecular dynamic simulation; molecular interactions; molecularly imprinted polymers; solubility; stability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Eersels K., Lieberzeit P., Wagner P. A Review on Synthetic Receptors for Bioparticle Detection Created by Surface-Imprinting Techniques—From Principles to Applications. ACS Sens. 2016;1:1171–1187. doi: 10.1021/acssensors.6b00572. - DOI
LinkOut - more resources
Full Text Sources

