Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review
- PMID: 39204573
- PMCID: PMC11360002
- DOI: 10.3390/polym16162353
Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review
Abstract
Intumescent fire-retardant coatings, which feature thinner layers and good decorative effects while significantly reducing heat transfer and air dispersion capabilities, are highly attractive for fire safety applications due to their effective prevention of material combustion and protection of materials. Particularly, the worldwide demand for improved environmental protection requirements has given rise to the production of waterborne intumescent fire-retardant polymer composite coatings, which are comparable to or provide more advantages than solvent-based intumescent fire-retardant polymer composite coatings in terms of low cost, reduced odor, and minimal environmental and health hazards. However, there is still a lack of a comprehensive and in-depth overview of waterborne intumescent fire-retardant polymer composite coatings. This review aims to systematically and comprehensively discuss the composition, the flame retardant and heat insulation mechanisms, and the practical applications of waterborne intumescent fire-retardant polymer composite coatings. Finally, some key challenges associated with waterborne intumescent fire-retardant polymer composite coatings are highlighted, following which future perspectives and opportunities are proposed.
Keywords: environmental protection; fire retardancy; intumescence; polymer; safety; waterborne coating.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

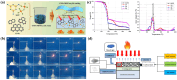

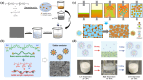









References
-
- Zhan W., Li L., Chen L., Kong Q., Chen M., Chen C., Zhang Q., Jiang J. Biomaterials in Intumescent Fire-Retardant Coatings: A Review. Prog. Org. Coat. 2024;192:108483. doi: 10.1016/j.porgcoat.2024.108483. - DOI
-
- Gu J., Zhang G., Dong S., Zhang Q., Kong J. Study on Preparation and Fire-Retardant Mechanism Analysis of Intumescent Flame-Retardant Coatings. Surf. Coat. Technol. 2007;201:7835–7841. doi: 10.1016/j.surfcoat.2007.03.020. - DOI
-
- Yan L., Xu Z., Wang X. Influence of Nano-Silica on the Flame Retardancy and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings. Prog. Org. Coat. 2017;112:319–329. doi: 10.1016/j.porgcoat.2017.07.017. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

