Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics
- PMID: 39204611
- PMCID: PMC11359314
- DOI: 10.3390/plants13162175
Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics
Abstract
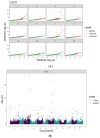
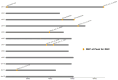
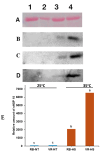
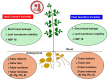
High temperatures during the crop growing season are becoming more frequent and unpredictable, resulting in reduced crop productivity and quality. Heat stress disrupts plant metabolic processes that affect cell membrane composition and integrity. Cell membrane permeability, ion leakage, and heat shock proteins have been evaluated to screen for heat tolerance in plants. In potatoes, it is unclear whether leaf membrane stability under heat stress is correlated with underground tuber productivity and quality. The main goal of this study was to evaluate if leaf membrane relative electrolyte conductivity (REC) under high temperatures could be used to identify heat-tolerant potato genotypes. Electrolyte leakage assays, correlation estimations, and genome-wide association studies were carried out in 215 genotypes. Expression levels of small heat shock protein 18 (sHSP18) were evaluated in the heat-sensitive potato variety Russet Burbank and compared with those of the heat-tolerant variety Vanguard Russet using Western blotting. Significant differences were observed among genotypes for leaf membrane REC under extreme heat (50°C); REC values ranged from 47.0-99.5%. Leaf membrane REC was positively correlated with tuber external and internal defects and negatively correlated with yield. REC was negatively correlated with the content of several tuber minerals, such as nitrogen, magnesium, and manganese. Eleven quantitative trait loci (QTLs) were identified for leaf membrane REC, explaining up to 13.8% of the phenotypic variance. Gene annotation in QTL areas indicated associations with genes controlling membrane solute transport and plant responses to abiotic stresses. Vanguard Russet had lower leaf REC and higher expression of sHSP18 under high-temperature stress. Our findings indicate that leaf membrane REC under high temperatures can be used as an indicator of potato heat tolerance.
Keywords: Solanum tuberosum; abiotic stress; heat shock proteins; heat stress; relative electrolyte conductivity.
Conflict of interest statement
The authors declare that the research was conducted without commercial or financial relationships that could create a conflict of interest.
Figures





Similar articles
-
Assessing heat tolerance in potatoes: Responses to stressful Texas field locations and controlled contrasting greenhouse conditions.Front Plant Sci. 2024 May 13;15:1364244. doi: 10.3389/fpls.2024.1364244. eCollection 2024. Front Plant Sci. 2024. PMID: 38803598 Free PMC article.
-
Development of an in vitro Microtuberization and Temporary Immersion Bioreactor System to Evaluate Heat Stress Tolerance in Potatoes (Solanum tuberosum L.).Front Plant Sci. 2021 Aug 11;12:700328. doi: 10.3389/fpls.2021.700328. eCollection 2021. Front Plant Sci. 2021. PMID: 34456944 Free PMC article.
-
Genetic Basis of Potato Tuber Defects and Identification of Heat-Tolerant Clones.Plants (Basel). 2024 Feb 23;13(5):616. doi: 10.3390/plants13050616. Plants (Basel). 2024. PMID: 38475462 Free PMC article.
-
Heat stress tolerance in peas (Pisum sativum L.): Current status and way forward.Front Plant Sci. 2023 Jan 17;13:1108276. doi: 10.3389/fpls.2022.1108276. eCollection 2022. Front Plant Sci. 2023. PMID: 36733601 Free PMC article. Review.
-
Mechanistic Concept of Physiological, Biochemical, and Molecular Responses of the Potato Crop to Heat and Drought Stress.Plants (Basel). 2022 Oct 26;11(21):2857. doi: 10.3390/plants11212857. Plants (Basel). 2022. PMID: 36365310 Free PMC article. Review.
References
-
- Lal M.K., Tiwari R.K., Kumar A., Dey A., Kumar R., Kumar D., Jaiswal A., Changan S.S., Raigond P., Dutt S., et al. Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants. 2022;11:2857. doi: 10.3390/plants11212857. - DOI - PMC - PubMed
-
- Savic J., Dragićević I., Pantelic D., Oljaca J., Momčilović I. Expression of small heat shock proteins and heat tolerance in potato (Solanum tuberosum L.) Arch. Biol. Sci. 2012;64:135–144. doi: 10.2298/ABS1201135S. - DOI
-
- Tiwari R., Lal M., Naga K., Kumar R., Chourasia K., Shivaramu S., Kumar D., Sharma S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020;272:109592. doi: 10.1016/j.scienta.2020.109592. - DOI
-
- Hasanuzzaman M., Bhuyan M.H.M.B., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. - DOI - PMC - PubMed
-
- ElBasyoni I., Saadalla M., Baenziger S., Bockelman H., Morsy S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability. 2017;9:1606. doi: 10.3390/su9091606. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

