Bedside Magnetocardiography with a Scalar Sensor Array
- PMID: 39205098
- PMCID: PMC11359538
- DOI: 10.3390/s24165402
Bedside Magnetocardiography with a Scalar Sensor Array
Abstract
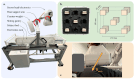
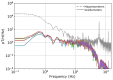
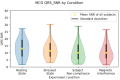
Decades of research have shown that magnetocardiography (MCG) has the potential to improve cardiac care decisions. However, sensor and system limitations have prevented its widespread adoption in clinical practice. We report an MCG system built around an array of scalar, optically pumped magnetometers (OPMs) that effectively rejects ambient magnetic interference without magnetic shielding. We successfully used this system, in conjunction with custom hardware and noise rejection algorithms, to record magneto-cardiograms and functional magnetic field maps from 30 volunteers in a regular downtown office environment. This demonstrates the technical feasibility of deploying our device architecture at the point-of-care, a key step in making MCG usable in real-world settings.
Keywords: magnetocardiography; medical devices; quantum sensors.
Conflict of interest statement
All authors were employed by SandboxAQ for the duration of the work presented.
Figures

References
-
- Khan M.A., Sun J., Li B., Przybysz A., Kosel J. Magnetic sensors-A review and recent technologies. Eng. Res. Express. 2021;3:022005. doi: 10.1088/2631-8695/ac0838. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

