Engineering of RNase P Ribozymes for Therapy against Human Cytomegalovirus Infection
- PMID: 39205170
- PMCID: PMC11360822
- DOI: 10.3390/v16081196
Engineering of RNase P Ribozymes for Therapy against Human Cytomegalovirus Infection
Abstract
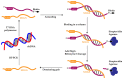
Nucleic acid-based gene interference and editing strategies, such as antisense oligonucleotides, ribozymes, RNA interference (RNAi), and CRISPR/Cas9 coupled with guide RNAs, are exciting research tools and show great promise for clinical applications in treating various illnesses. RNase P ribozymes have been engineered for therapeutic applications against human viruses such as human cytomegalovirus (HCMV). M1 ribozyme, the catalytic RNA subunit of RNase P from Escherichia coli, can be converted into a sequence-specific endonuclease, M1GS ribozyme, which is capable of hydrolyzing an mRNA target base-pairing with the guide sequence. M1GS RNAs have been shown to hydrolyze essential HCMV mRNAs and block viral progeny production in virus-infected cell cultures. Furthermore, RNase P ribozyme variants with enhanced hydrolyzing activity can be generated by employing in vitro selection procedures and exhibit better ability in suppressing HCMV gene expression and replication in cultured cells. Additional studies have also examined the antiviral activity of RNase P ribozymes in mice in vivo. Using cytomegalovirus infection as an example, this review summarizes the principles underlying RNase P ribozyme-mediated gene inactivation, presents recent progress in engineering RNase P ribozymes for applications in vitro and in mice, and discusses the prospects of using M1GS technology for therapeutic applications against HCMV as well as other pathogenic viruses.
Keywords: HCMV; RNase P; antisense; antivirals; catalytic RNA; gene targeting; ribozyme.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical