A small TAT-TrkB peptide prevents BDNF receptor cleavage and restores synaptic physiology in Alzheimer's disease
- PMID: 39205389
- PMCID: PMC11489560
- DOI: 10.1016/j.ymthe.2024.08.022
A small TAT-TrkB peptide prevents BDNF receptor cleavage and restores synaptic physiology in Alzheimer's disease
Erratum in
-
A small TAT-TrkB peptide prevents BDNF receptor cleavage and restores synaptic physiology in Alzheimer's disease.Mol Ther. 2025 Jan 8;33(1):421. doi: 10.1016/j.ymthe.2024.12.005. Epub 2024 Dec 12. Mol Ther. 2025. PMID: 39672159 Free PMC article. No abstract available.
Abstract
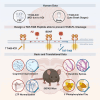
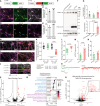
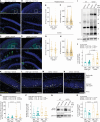
In Alzheimer's disease (AD), amyloid β (Aβ)-triggered cleavage of TrkB-FL impairs brain-derived neurotrophic factor (BDNF) signaling, thereby compromising neuronal survival, differentiation, and synaptic transmission and plasticity. Using cerebrospinal fluid and postmortem human brain samples, we show that TrkB-FL cleavage occurs from the early stages of the disease and increases as a function of pathology severity. To explore the therapeutic potential of this disease mechanism, we designed small TAT-fused peptides and screened their ability to prevent TrkB-FL receptor cleavage. Among these, a TAT-TrkB peptide with a lysine-lysine linker prevented TrkB-FL cleavage both in vitro and in vivo and rescued synaptic deficits induced by oligomeric Aβ in hippocampal slices. Furthermore, this TAT-TrkB peptide improved the cognitive performance, ameliorated synaptic plasticity deficits and prevented Tau pathology progression in vivo in the 5XFAD mouse model of AD. No evidence of liver or kidney toxicity was found. We provide proof-of-concept evidence for the efficacy and safety of this therapeutic strategy and anticipate that this TAT-TrkB peptide has the potential to be a disease-modifying drug that can prevent and/or reverse cognitive deficits in patients with AD.
Keywords: Alzheimer’s disease; BDNF; TAT peptide; TAT-TrkB; TrkB receptor; amyloid β; drug screening; hippocampal plasticity; learning; memory; protein cleavage.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.F.-G., M.J.D., A.J.-S., C.B.D., and A.M.S. are authors of a patent (application no. PCT/PT2021/050011; priority date: April 1, 2020) concerning the prevention of TrkB-FL cleavage as a therapeutic strategy.
Figures





References
-
- Goedert M., Wischik C.M., Crowther R.A., Walker J.E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

