IL-2-armored peptide-major histocompatibility class I bispecific antibodies redirect antiviral effector memory CD8+ T cells to induce potent anti-cancer cytotoxic activity with limited cytokine release
- PMID: 39205483
- PMCID: PMC11364066
- DOI: 10.1080/19420862.2024.2395499
IL-2-armored peptide-major histocompatibility class I bispecific antibodies redirect antiviral effector memory CD8+ T cells to induce potent anti-cancer cytotoxic activity with limited cytokine release
Abstract
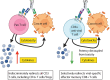
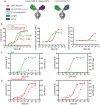
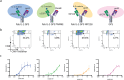
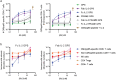
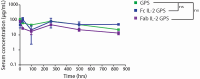
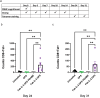
T cell engagers (TCEs) are becoming an integral class of biological therapeutic owing to their highly potent ability to eradicate cancer cells. Nevertheless, the widespread utility of classical CD3-targeted TCEs has been limited by narrow therapeutic index (TI) linked to systemic CD4+ T cell activation and aberrant cytokine release. One attractive approach to circumvent the systemic activation of pan CD3+ T cells and reduce the risk of cytokine release syndrome is to redirect specific subsets of T cells. A promising strategy is the use of peptide-major histocompatibility class I bispecific antibodies (pMHC-IgGs), which have emerged as an intriguing modality of TCE, based on their ability to selectively redirect highly reactive viral-specific effector memory cytotoxic CD8+ T cells to eliminate cancer cells. However, the relatively low frequency of these effector memory cells in human peripheral blood mononuclear cells (PBMCs) may hamper their redirection as effector cells for clinical applications. To mitigate this potential limitation, we report here the generation of a pMHC-IgG derivative known as guided-pMHC-staging (GPS) carrying a covalent fusion of a monovalent interleukin-2 (IL-2) mutein (H16A, F42A). Using an anti-epidermal growth factor receptor (EGFR) arm as a proof-of-concept, tumor-associated antigen paired with a single-chain HLA-A *02:01/CMVpp65 pMHC fusion moiety, we demonstrate in vitro that the IL-2-armored GPS modality robustly expands CMVpp65-specific CD8+ effector memory T cells and induces potent cytotoxic activity against target cancer cells. Similar to GPS, IL-2-armored GPS molecules induce modulated T cell activation and reduced cytokine release profile compared to an analogous CD3-targeted TCE. In vivo we show that IL-2-armored GPS, but not the corresponding GPS, effectively expands grafted CMVpp65 CD8+ T cells from unstimulated human PBMCs in an NSG mouse model. Lastly, we demonstrate that the IL-2-armored GPS modality exhibits a favorable developability profile and monoclonal antibody-like pharmacokinetic properties in human neonatal Fc receptor transgenic mice. Overall, IL-2-armored GPS represents an attractive approach for treating cancer with the potential for inducing vaccine-like antiviral T cell expansion, immune cell redirection as a TCE, and significantly widened TI due to reduced cytokine release.
Keywords: Antibody; CRS; MHC; T cell engager; TCE; antibody engineering; bispecific; cancer; cytokine release syndrome; major histocompatibility complex.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- The antibody society. Therapeutic monoclonal antibodies approved or in regulatory review. 2024 Aug 16; www.antibodysociety.org/antibody-therapeutics-product-data.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
