Lactobacillus acidophilus ameliorates cholestatic liver injury through inhibiting bile acid synthesis and promoting bile acid excretion
- PMID: 39205654
- PMCID: PMC11364073
- DOI: 10.1080/19490976.2024.2390176
Lactobacillus acidophilus ameliorates cholestatic liver injury through inhibiting bile acid synthesis and promoting bile acid excretion
Abstract
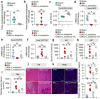
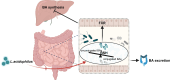
Gut microbiota dysbiosis is involved in cholestatic liver diseases. However, the mechanisms remain to be elucidated. The purpose of this study was to examine the effects and mechanisms of Lactobacillus acidophilus (L. acidophilus) on cholestatic liver injury in both animals and humans. Bile duct ligation (BDL) was performed to mimic cholestatic liver injury in mice and serum liver function was tested. Gut microbiota were analyzed by 16S rRNA sequencing. Fecal bacteria transplantation (FMT) was used to evaluate the role of gut microbiota in cholestasis. Bile acids (BAs) profiles were analyzed by targeted metabolomics. Effects of L. acidophilus in cholestatic patients were evaluated by a randomized controlled clinical trial (NO: ChiCTR2200063330). BDL induced different severity of liver injury, which was associated with gut microbiota. 16S rRNA sequencing of feces confirmed the gut flora differences between groups, of which L. acidophilus was the most distinguished genus. Administration of L. acidophilus after BDL significantly attenuated hepatic injury in mice, decreased liver total BAs and increased fecal total BAs. Furthermore, after L. acidophilus treatment, inhibition of hepatic Cholesterol 7α-hydroxylase (CYP7α1), restored ileum Fibroblast growth factor 15 (FGF15) and Small heterodimer partner (SHP) accounted for BAs synthesis decrease, whereas enhanced BAs excretion was attributed to the increase of unconjugated BAs by enriched bile salt hydrolase (BSH) enzymes in feces. Similarly, in cholestasis patients, supplementation of L. acidophilus promoted the recovery of liver function and negatively correlated with liver function indicators, possibly in relationship with the changes in BAs profiles and gut microbiota composition. L. acidophilus treatment ameliorates cholestatic liver injury through inhibited hepatic BAs synthesis and enhances fecal BAs excretion.
Keywords: Cholestatic liver injury; Lactobacillus; gut microbiota.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
