Local Disease-Free Survival and Disease-Free Survival in Locally Advanced Cervical Cancer Diagnosed and Treated in Bihor County, Romania
- PMID: 39205752
- PMCID: PMC11350524
- DOI: 10.7759/cureus.65629
Local Disease-Free Survival and Disease-Free Survival in Locally Advanced Cervical Cancer Diagnosed and Treated in Bihor County, Romania
Abstract
Introduction: Cervical cancer is the fourth most dangerous malignancy worldwide in women and is diagnosed at the advanced stages in most cases. Oncological and surgical modalities when precisely employed together can prove to be helpful for determining the proper diagnosis and treatment strategies. Neoadjuvant chemotherapy (NACT) has been found to have a role in reducing tumor size and has evolved as a treatment regimen for locally advanced cervical cancer (LACC). The present study aimed to analyze the treatment strategies either with neoadjuvant platinum-based chemotherapy (NACT) administration or not and pathological responses in patients with LACC.
Methods: We reviewed 100 patients of LACC from October 2018 to December 2022 at Bihor County Emergency Clinical Hospital. About 43 patients underwent radiation therapy in addition to NACT administration (NACT+/other) and 57 underwent other treatment regimens without neoadjuvant treatment (NACT-/ other). Various demographic parameters, FIGO staging, histological status, surgical interventions, and survival rate (local disease-free survival (LDFS) and disease-free survival (DFS)) were accessed in both groups. Statistical analysis was performed to analyze the significance of various parameters studied.
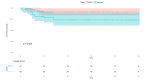
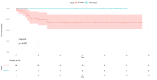
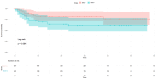
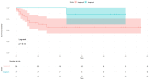
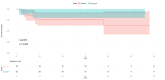
Results: The mean age range of the studied sample was 57.05 ± 12.51 in NACT+/other and 60.4±12.32 in NACT-/other. Among 100 patients, 90 cases of squamous carcinoma, eight of adenocarcinoma, and two cases of adenosquamous carcinoma were analyzed and treated. At stage IIIC1, 11 patients were accessed while 15 patients were at clinical stage IIIC2, and among these, 25.58% received neoadjuvant oncological treatment and very limited mediastinal disease. DFS rates are greater in the patients who have undergone surgery in the NACT+/other group, while in the LDFS, there is better survival in the case of surgery without any NACT treatment (NACT-/other).
Conclusions: The effect of NACT can be suggested as another important treatment strategy and result in a good response in terms of DFS and LDFS in patients with LACC. This approach aims to reduce tumor size preoperatively, facilitating surgical removal and potentially improving patient outcomes compared to other treatment modalities. Thus, it can be concluded that NACT may be considered an important strategy to be opted for the treatment of LACC.
Keywords: cervical cancer; disease-free survival; local disease-free survival; locally advanced cervical cancer (lacc); neoadjuvant chemotherapy; surgery; tumor grading.
Copyright © 2024, Molnar et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. The Ethical Council of the Bihor County Emergency Clinical Hospital issued approval 43608/14.12.23. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Cancer statistics for the year 2020: An overview. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Int J Cancer. 2021 - PubMed
-
- International Agency for Research on Cancer. Globocan 2018: Romania. 2020. https://gco.iarc.fr/today/data/ https://gco.iarc.fr/today/data/
LinkOut - more resources
Full Text Sources
