Alpha-Lipoic Acid Ameliorates Impaired Steroidogenesis in Human Granulosa Cells Induced by Advanced Glycation End-Products
- PMID: 39205823
- PMCID: PMC11347593
- DOI: 10.30476/IJMS.2023.99512.3168
Alpha-Lipoic Acid Ameliorates Impaired Steroidogenesis in Human Granulosa Cells Induced by Advanced Glycation End-Products
Abstract
Background: Ovarian granulosa cells (GCs) are essential for follicular development. Ovarian advanced glycation end-products (AGEs) accumulation is related to GCs dysfunction. Alpha-lipoic acid (ALA) illustrates therapeutic capabilities for infertility-related disorders. Therefore, this study assessed the effects of ALA on AGEs-induced GCs hormonal dysfunction.
Methods: The study was conducted from October 2021 to September 2022 at the Department of Medical Genetics, Shiraz University of Medical Sciences. Isolated GCs (n=50) were divided into control, human glycated albumin (HGA), HGA+ALA, and ALA treatments. Steroidogenic enzymes and AGE receptor (RAGE) genes were assessed by qRT-PCR. Steroid hormones and RAGE protein were evaluated using ELISA and Western blotting. Data were analyzed using GraphPad Prism software (ver. 9), and P<0.05 was considered significant.
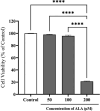
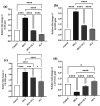
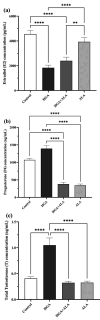
Results: Our findings showed that HGA treatment significantly (P=0.0001) increased RAGE (by 140.66%), STAR (by 117.65%), 3β-HSD (by 165.68%), and 17β-HSD (by 122.15%) expression, while it decreased CYP19A1 (by 68.37%) expression. RAGE protein level (by 267.10%) was also increased in HGA-treated GCs. A significant decrease in estradiol (by 59.66%) and a slight and sharp elevation in progesterone (by 30.40%) and total testosterone (by 158.24%) levels was also observed. ALA treatment ameliorated the HGA-induced changes in steroidogenic enzyme mRNA levels (P=0.001) and steroid hormone secretion (P=0.010).
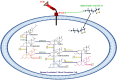
Conclusion: This work shows that ALA therapy likely corrects hormonal dysfunctions caused by AGEs in luteinized GCs. This effect is probably achieved by decreased RAGE expression. Clinical research is needed to understand how AGEs and ALA interact in the ovary, which might lead to a more targeted ovarian dysfunction therapy.
Keywords: Advanced glycation end-products; Alpha-lipoic acid; Gonadal steroid hormones; Granulosa cells; Humans.
Copyright: © Iranian Journal of Medical Sciences.
Conflict of interest statement
None declared.
Figures


References
-
- Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, et al. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol. 2020;197:105521. doi: 10.1016/j.jsbmb.2019.105521. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
