Long noncoding RNA MALAT1 mediates fibrous topography-driven pathologic calcification through trans-differentiation of myoblasts
- PMID: 39205874
- PMCID: PMC11357808
- DOI: 10.1016/j.mtbio.2024.101182
Long noncoding RNA MALAT1 mediates fibrous topography-driven pathologic calcification through trans-differentiation of myoblasts
Abstract
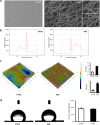
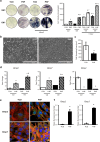
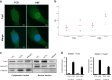
Prosthesis-induced pathological calcification is a significant challenge in biomaterial applications and is often associated with various reconstructive medical procedures. It is uncertain whether the fibrous extracellular matrix (ECM) adjacent to biomaterials directly triggers osteogenic trans-differentiation in nearby cells. To investigate this possibility, we engineered a heterogeneous polystyrene fibrous matrix (PSF) designed to mimic the ECM. Our findings revealed that the myoblasts grown on this PSF acquired osteogenic properties, resulting in mineralization both in vitro and in vivo. Transcriptomic analyses indicated a notable upregulation in the expression of the long noncoding RNA metastsis-associated lung adenocarcinoma transcript 1 (Malat1) in the C2C12 myoblasts cultured on PSF. Intriguingly, silencing Malat1 curtailed the PSF-induced mineralization and downregulated the expression of bone morphogenetic proteins (Bmps) and osteogenic markers. Further, we found that PSF prompted the activation of Yap1 signaling and epigenetic modifications in the Malat1 promoter, crucial for the expression of Malat1. These results indicate that the fibrous matrix adjacent to biomaterials can instigate Malat1 upregulation, subsequently driving osteogenic trans-differentiation in myoblasts and ectopic calcification through its transcriptional regulation of osteogenic genes, including Bmps. Our findings point to a novel therapeutic avenue for mitigating prosthesis-induced pathological calcification, heralding new possibilities in the field of biomaterial-based therapies.
Keywords: Fibrous ECM mimicry; Malat1; Prosthesis-induced pathologic calcification; Trans-differentiation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Anderson H.C. Mechanisms of pathologic calcification. Rheum Dis Clin North Am. 1988;14:303–319. - PubMed
LinkOut - more resources
Full Text Sources

