Fast formation and maturation enhancement of human liver organoids using a liver-organoid-on-a-chip
- PMID: 39206088
- PMCID: PMC11349704
- DOI: 10.3389/fcell.2024.1452485
Fast formation and maturation enhancement of human liver organoids using a liver-organoid-on-a-chip
Abstract
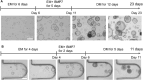
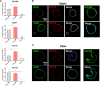
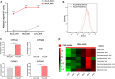
Background: Spatial and functional hepatic zonation, established by the heterogeneous tissue along the portal-central axis of the liver, is important for ensuring optimal liver function. Researchers have attempted to develop reliable hepatic models to mimic the liver microenvironment and analyze liver function using hepatocytes cultured in the developed systems. However, mimicking the liver microenvironment in vitro remains a great challenge owing to the lack of perfusable vascular networks in the model systems and the limitation in maintaining hepatocyte function over time. Methods: In this study, we established a microphysiological system that operated under continuous flush medium flow, thereby allowing the supply of nutrients and oxygen to liver organoids and the removal of waste and release of cytokines therefrom, similar to the function of blood vessels. Results: The application of microphysiological system to organoid culture was advantageous for reducing the differentiation time and enhancing the functional maturity of human liver organoid. Conclusion: Hence, our microphysiological culture system might open the possibility of the miniaturized liver model system into a single device to enable more rational in vitro assays of liver response.
Keywords: 3D cell culture; hepatic models; liver maturation; liver microenvironment; microphysiological system (MPS).
Copyright © 2024 Byeon, Jung, Han, Son and Jeong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Balakrishnan S., Suma M. S., Raju S. R., Bhargav S. D., Arunima S., Das S., et al. (2015). A scalable perfusion culture system with miniature peristaltic pumps for live-cell imaging assays with provision for microfabricated scaffolds. Biores Open Access 4, 343–357. 10.1089/biores.2015.0024 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

