CD4+ tumor-infiltrating lymphocytes secreting T cell-engagers induce regression of autologous patient-derived non-small cell lung cancer xenografts
- PMID: 39206095
- PMCID: PMC11352715
- DOI: 10.1080/2162402X.2024.2392897
CD4+ tumor-infiltrating lymphocytes secreting T cell-engagers induce regression of autologous patient-derived non-small cell lung cancer xenografts
Abstract
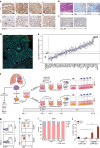
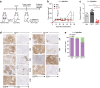
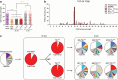
Adoptive transfer of tumor-infiltrating lymphocytes (TIL) has shown remarkable results in melanoma, but only modest clinical benefits in other cancers, even after TIL have been genetically modified to improve their tumor homing, cytotoxic potential or overcome cell exhaustion. The required ex vivo TIL expansion process may induce changes in the T cell clonal composition, which could likely compromise the tumor reactivity of TIL preparations and ultimately the success of TIL therapy. A promising approach based on the production of bispecific T cell-engagers (TCE) by engineered T cells (STAb-T therapy) improves the efficacy of current T cell redirection strategies against tumor-associated antigens in hematological tumors. We studied the TCRβ repertoire in non-small cell lung cancer (NSCLC) tumors and in ex vivo expanded TIL from two unrelated patients. We generated TIL secreting anti-epidermal growth factor receptor (EGFR) × anti-CD3 TCE (TILSTAb) and tested their antitumor efficacy in vitro and in vivo using a NSCLC patient-derived xenograft (PDX) model in which tumor fragments and TIL from the same patient were transplanted into hIL-2 NOG mice. We confirmed that the standard TIL expansion protocol promotes the loss of tumor-dominant T cell clones and the overgrowth of virus-reactive TCR clonotypes that were marginally detectable in primary tumors. We demonstrated the antitumor activity of TILSTAb both in vitro and in vivo when administered intratumorally and systemically in an autologous immune-humanized PDX EGFR+ NSCLC mouse model, where tumor regression was mediated by TCE-redirected CD4+ TIL bearing non-tumor dominant clonotypes.
Keywords: Adoptive cell therapy; bispecific T cell-engagers; cytotoxic CD4+ TIL; solid tumors; tumor-infiltrating lymphocytes.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
B.B., L.P-A and L.A-V are co-founders and shareholders of STAb Therapeutics, a spin-off company from the imas12. B.B. and L.A-V are inventors on the patent EP21708942 pending. L.D-A. and L.A-V are inventors on the patent EP23383410.0 pending. L.A-V is cofounder and shareholder of Leadartis, a company focused on unrelated interest. L.A-V reports speaker honoraria from MSD, Merck KGaA, BMS, Janssen, GSK, and Miltenyi, and receives grant support from Merck KGaA, all outside the submitted work. J.Z. has served as a consultant for Sanofi, Pfizer, Astra Zeneca, BMS, Novartis, NanoString, and Guardant Health; reports speaker honoraria from Pfizer, BMS, Roche, Astra Zeneca, NanoString and Guardant Health; and receives grant support from Astra Zeneca, Roche, and BMS, all outside the submitted work. L.P-A. is shareholder of Altum Squencing and STAb Therapeutics, has served as a consultant for Lilly, MSD, Roche, Pharmamar, Merck KGaA, Astra Zeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati, GSK, Janssen, Takeda, Regeneron, and Sanofi. L.P-A. received grant support from MSD, Astra Zeneca, BMS, Pfizer and Pharmamar, all outside the submitted work. R.T. and E.P. are consultants for the company NISOLAB.
Figures

References
-
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. - DOI - PubMed
-
- Chesney J, Lewis KD, Kluger H, Hamid O, Whitman E, Thomas S, Wermke M, Cusnir M, Domingo-Musibay E, Phan GQ, et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J Immunother Cancer. 2022;10(12). doi: 10.1136/jitc-2022-005755. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
