Overexpression of CCL-20 and CXCL-8 genes enhances tumor escape and resistance to cemiplimab, a programmed cell death protein-1 (PD-1) inhibitor, in patients with locally advanced and metastatic cutaneous squamous cell carcinoma
- PMID: 39206096
- PMCID: PMC11352706
- DOI: 10.1080/2162402X.2024.2388315
Overexpression of CCL-20 and CXCL-8 genes enhances tumor escape and resistance to cemiplimab, a programmed cell death protein-1 (PD-1) inhibitor, in patients with locally advanced and metastatic cutaneous squamous cell carcinoma
Abstract
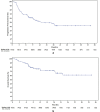
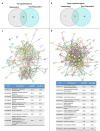
Cemiplimab has demonstrated relevant clinical activity in cutaneous squamous cell carcinoma (cSCC) but mechanisms of primary and acquired resistance to immunotherapy are still unknown. We collected clinical data from locally advanced and/or metastatic cSSC patients treated with cemiplimab in two Italian University centers. In addition, gene expression analysis by using Nanostring Technologies platform to evaluate 770 cancer- and immune-related genes on 20 tumor tissue samples (9 responders and 11 non-responders to cemiplimab) was performed. We enrolled 81 patients with a median age of 82 years. After 16.4 months of median follow-up, 12- and 24-months PFS were 53% and 42%, respectively; while 12- and 24-months OS were 71% and 61%, respectively. Treatment was well tolerated. Overall response rate (ORR) was 58%, with a disease control rate (DCR) of 77.8%. The difference between genes expressed in responder versus non-responder patient samples was substantial, particularly for genes involved in immune system regulation. Cemiplimab-resistant tumors were associated with over-expression of CCL-20 and CXCL-8. Cemiplimab confirmed efficacy and safety data in real-life cSCC patients. Overexpression of CCL-20 and CXCL-8 could represent biomarkers of lack of response to immunotherapy.
Keywords: Cemiplimab; cSCC; cutaneous squamous cell carcinoma; gene expression profiling; immunotherapy; nanostring; real-world data.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
S.N. had travel grants from Amgen, Merck outside of the submitted works. D.C. had travel support from Sanofi, BMS, Merck serono outside of the submitted works. F.C. was advisory board for Amgen, Servier, MSD, Merck, Roche, Pfizer, Bayer, Pierre Fabre, Eisai outside of the submitted work. T.T. was advisory board for Amgen, MSD, Pierre Fabre, Roche, Merck outside of the submitted work. All remaining authors have no competing interests.
Figures



References
-
- Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, Dreno B, Fargnoli MC, Forsea AM, Frenard C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 1. epidemiology, diagnostics and prevention. Eur J Cancer. 2020;128:60–11. doi:10.1016/j.ejca.2020.01.007. - DOI - PubMed
-
- Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, Schadendorf D, Dunn LA, Hernandez-Aya L, Chang ALS, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020;8(1):e000775. doi:10.1136/jitc-2020-000775. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
