Fer governs mTORC1 regulating pathways and sustains viability of pancreatic ductal adenocarcinoma cells
- PMID: 39206154
- PMCID: PMC11349523
- DOI: 10.3389/fonc.2024.1427029
Fer governs mTORC1 regulating pathways and sustains viability of pancreatic ductal adenocarcinoma cells
Abstract
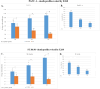
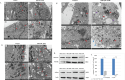
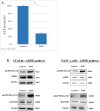
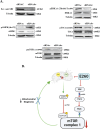
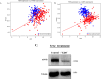
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers with a high percentage of morbidity. The deciphering and identification of novel targets and tools for intervening with its adverse progression are therefore of immense importance. To address this goal we adopted a specific inhibitor of the intracellular tyrosine kinase Fer, whose expression level is upregulated in PDAC tumors, and is associated with poor prognosis of patients. Subjecting PDAC cells to the E260-Fer inhibitor, unraveled its simultaneous effects on the mitochondria, and on a non-mitochondrial ERK1/2 regulatory cascade. E260 caused severe mitochondrial deformation, resulting in cellular- aspartate and ATP depletion, and followed by the activation of the metabolic sensor AMPK. This led to the phosphorylation and deactivation of the bona fide AMPK substrate, RAPTOR, which serves as a positive regulator of the mTORC1 metabolic hub. Accordingly, this resulted in the inhibition of the mTORC1 activity. In parallel, E260 downregulated the activation state of the ERK1/2 kinases, and their ability to neutralize the mTORC1 suppressor TSC2, thereby accentuating the inhibition of mTORC1. Importantly, both activation of AMPK and downregulation of ERK1/2 and mTORC1 were also achieved upon the knockdown of Fer, corroborating the regulatory role of Fer in these processes. Concomitantly, in PDAC tumors and not in healthy pancreatic tissues, the expression levels of Fer demonstrate moderate but statistically significant positive correlation with the expression levels of mTOR and its downstream effector LARP1. Finally, targeting the Fer driven activation of mTORC1, culminated in necrotic death of the treated PDAC cells, envisaging a new intervention tool for the challenging PDAC disease.
Keywords: AMPK; E260; Fer; mTORC1; mitochondria; pancreatic ductal adenocarcinoma.
Copyright © 2024 Schrier, Slotki-Itzchakov, Elkis, Most-Menachem, Adato, Fitoussi-Allouche, Shpungin, Unger and Nir.
Conflict of interest statement
SS, and UN hold patents related to Fer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Letwin K, Yee SP, Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using antiphosphotyrosine antibody. Oncogene. (1988) 3:621–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

