IgA vasculitis induced by carboplatin + nab-paclitaxel + pembrolizumab in a patient with advanced lung squamous cell carcinoma: a case report
- PMID: 39206190
- PMCID: PMC11349625
- DOI: 10.3389/fimmu.2024.1370972
IgA vasculitis induced by carboplatin + nab-paclitaxel + pembrolizumab in a patient with advanced lung squamous cell carcinoma: a case report
Abstract
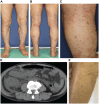
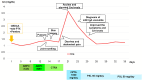
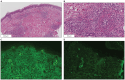
A 73-year-old man with lung squamous cell carcinoma was administered carboplatin + nab-paclitaxel + pembrolizumab for four cycles. Subsequently, he presented with multiple purpuras on his extremities, joint swelling on his fingers, abdominal pain, and diarrhea, accompanied by acute kidney injury (AKI), increased proteinuria, hematuria, and elevated C-reactive protein levels. Skin biopsy showed leukocytoclastic vasculitis as well as IgA and C3 deposition in the vessel walls. Based on these findings, the patient was diagnosed with IgA vasculitis as an immune-related adverse event (irAE) induced by carboplatin + nab-paclitaxel + pembrolizumab. After discontinuation of pembrolizumab and glucocorticoids, the symptoms immediately resolved. Regular monitoring of skin, blood tests, and urinalysis are necessary, and the possibility of irAE IgA vasculitis should be considered in cases of purpura and AKI during treatment with immune checkpoint inhibitors.
Keywords: IgA vasculitis; immune checkpoint inhibitor; immune-related adverse event; non-small-cell lung cancer; pembrolizumab.
Copyright © 2024 Terashima, Matsumoto, Ozaki, Nakagawa, Nakagome, Terasaki, Iida, Mitsugi, Kuramochi, Okada, Inoue, Matsuki, Kitagawa, Fukuizumi, Onda, Takeuchi, Miyanaga, Kasahara and Seike.
Conflict of interest statement
AM has received honoraria from AstraZeneca, Nippon Kayaku, Merck, Kyowa Kirin, and Pfizer. KK has received honoraria Chugai Pharmaceutical, Eli Lilly, and Bristol-Myers Squibb. MS has received grants and contracts from any entity from Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Nippon Boehringer Ingelheim, Nippon Kayaku, and Kyowa Hakko Kirin; honoraria from AstraZeneca, MSD K.K, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Pfizer, Novartis, Takeda Pharmaceutical, Kyowa Hakko Kirin, Nippon Kayaku, Daiichi-Sankyo Company, Merck Biopharma, and Amgen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

