Manganese boosts natural killer cell function via cGAS-STING mediated UTX expression
- PMID: 39206412
- PMCID: PMC11351689
- DOI: 10.1002/mco2.683
Manganese boosts natural killer cell function via cGAS-STING mediated UTX expression
Abstract
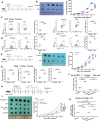
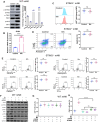
Natural killer (NK) cells play a crucial role in both innate immunity and the activation of adaptive immunity. The activating effect of Mn2+ on cyclic GMP-AMP(cGAS)-stimulator of interferon genes (STING signaling has been well known, but its effect on NK cells remains elusive. In this study, we identified the vital role of manganese (Mn2+) in NK cell activation. Mn2+ directly boosts cytotoxicity of NK cells and promotes the cytokine secretion by NK cells, thereby activating CD8+ T cells and enhancing their antitumor activity. Furthermore, Mn2+ can simultaneously activate NK-cell intrinsic cGAS and STING and consequently augment the expression of ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX to promote the responsiveness of NK cells. Our results contribute to a broader comprehension of how cGAS-STING regulates NK cells. As a potent agonist of cGAS-STING, Mn2+ provides a promising option for NK cell-based immunotherapy of cancers.
Keywords: UTX; antitumor immunity; cGAS–STING; manganese; natural killer cells.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures




References
-
- Mellman I, Chen DS, Powles T, Turley SJ. The cancer‐immunity cycle: indication, genotype, and immunotype. Immunity. 2023;56(10):2188‐2205. - PubMed
-
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1(1):41‐49. - PubMed
-
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
